
PC-BaX Server Edition 3.0 serial key or number

PC-BaX Server Edition 3.0 serial key or number
Frequently Asked Questions
Frequently Asked Questions

Do you have a question that is not answered below? Ask your question here.
Sign up for our newsletter!
General Questions
ATP Products
UltraSnap, SuperSnap, AquaSnap
How does bioluminescence work?
Bioluminecsence is the result of a biochemical reaction and is the science behind Hygiena ATP detection tests. The reaction includes the following elements:
- Luciferase enzyme- naturally occurring in fireflies, or synthetically manufactured
- Adenosine triphosphate (ATP) - the energy molecule of all living organisms
- Oxygen- a catalyst
- Luciferin- a molecule that undergoes a chemical charge when affixed by an enzyme
ATP + Luciferin + Luciferase + O2 == == Light output
The reaction occurs in two steps: The substrate combines with ATP and oxygen, which is controlled by the enzyme. The chemical energy in step 1 excites a specific molecule (the combination of Luciferin and Luciferase). The result is decay which is manifested as photon emission, or light production. The light is simply a by-product of the chemical reaction and does not depend on light.

What is ATP Monitoring?
ATP monitoring is a rapid testing method used by food and beverage processors to quickly assess the cleanliness of surfaces or liquid samples from such places as CIP systems. Adenosine Triphosphate (ATP) is present in all organic material and is the universal unit of energy used in all living cells. ATP is produced and/or broken down in metabolic processes in all living systems. Processes such as photosynthesis in plants, muscle contraction in humans, respiration in fungi, and fermentation in yeast are all driven by ATP. Therefore, most foods and microbial cells will contain some level of naturally occurring ATP.
Hygiena luminometers (in conjunction with ATP swabs) use bioluminescence to detect residual ATP as an indicator of surface cleanliness. The presence of ATP on a surface indicates improper cleaning and the presence of contamination, including food residue, allergens and/or bacteria. This implies a potential for the surface to harbor and support bacterial growth.
ATP monitoring is used in food and beverage facilities to confirm that ATP presence is eliminated or minimized by effective sanitation procedures. ATP monitoring prevents cross-contamination, ensures product integrity, potentially improves product shelf life, protects brand reputation, and complies with GMP standards and HACCP requirements.
How many food and beverage processing companies are doing ATP monitoring?
Nearly all food and beverage companies that process food with any water activity or can sustain organisms do ATP testing. Companies with highly processed materials like oil or companies dealing with products with no water activity like dry grains, sometimes will not do ATP testing. ATP testing is used around the globe and is a recognized tool by auditors for validating cleaning processes and complies with government and HACCP regulations.
Do all foods contain ATP?
Because ATP is present in all organic matter, all foods contain ATP at varying levels. This is why testing for ATP presence is the preferred way to measure the cleanliness of surfaces and water samples. Highly processes foods such as oil will have very little to no ATP.
Are there any studies or white papers on ATP testing?
You can find third party research, editorials, reports, and other documents via the resource library here. Many manufacturers offer seemingly scientific research supporting their own ATP systems. It is important for customers to understand that only objective studies conducted by independent laboratories should be accepted as scientific evidence of product performance. Silliker Laboratories, the leading international food testing and safety laboratory, conducted an independent study comparing five commercial ATP systems. This study found that Hygiena’s products offer superior linearity, sensitivity, repeatability, and accuracy. Request a copy here!
What is an RLU?
RLU stands for Relative Light Unit and is the unit of measure used in bioluminescence. When a test swab is activated, a bioluminescent reaction occurs, generating light output. Luminometers measure and quantify that light with an RLU output. Because manufacturers use different sensor technologies and algorithms for adding up the photons, RLU measurements will vary from system to system. However, because the ATP bioluminescence reaction is linear, the more ATP present means the more light will be present. This makes comparing systems easy. (Comparing RLU values is like comparing Fahrenheit and Celsius; they are two different scales for the same temperature.) For more information on comparing systems, click here.
The relationship between the amount of ATP on the sample and the RLU result reading on the luminometer is simple:
High contamination (improper cleaning) = Large amount of ATP = More light produced in reaction= High RLU reading on SystemSURE Plus or EnSURE

The RLU reading is directly proportional to the amount of ATP collected from the sample. A high RLU reading indicates a large amount of ATP at the test location. This in turn indicates improper cleaning and the presence of contaminants.
Cleaning properly results in less ATP at the location. Less ATP results in less light output during the bioluminescent reaction and consequently, a lower RLU reading.
Does a 0 (zero) RLU result indicate sterility?
Sterile is a term relating to a true microbiology test i.e., the absence of living bacteria. An ATP test detects all sources of ATP but cannot differentiate ATP from food or bacteria, thus a zero RLU result cannot be equated with sterility.
What is the limit of detection of Hygiena ATP tests?
The limit of detection depends on the test device and luminometer used:
- UltraSnap & SystemSURE Plus : 1 femtomole of ATP
- SuperSnap & SystemSURE Plus : <0.20 femtomoles of ATP
- SuperSnap & EnSURE : <0.10 femtomoles of ATP
How do I set my Pass and Fail limits for my ATP program?
All systems come preset with generic limits that can usually be applied to most processing facilities. These thresholds have been established over the years by companies doing ATP testing and supported by research papers on the science. These thresholds are typically a good starting point for companies new to ATP testing.
Click here for a video and step by step guide for setting up and determining RLU limits!
Can I have different limits for different locations?
Yes. Pass/Fail limits are 100% customizable to fit your facility and program needs. To adjust limits for specific locations, simply edit location information in SureTrend software.
Can I compare RLU measurement to CFU plate counts?
ATP systems with standard ATP test swabs detect the presence of ATP and cannot distinguish microbial ATP from other organic ATP left behind after cleaning. RLU results could potentially be microbial cells, organic ATP from product residue, or both. This makes comparing RLU to CFU difficult. Studies have shown that there is anywhere between 60-90% correlation between RLU and CFU readings depending on the environment in which the system is used.
The presence of ATP on the surface indicates that it has not been adequately cleaned. The primary purpose of cleaning is to remove product residue for product contact surfaces. Effective cleaning simultaneously removes the material capable of supporting microbial survival and growth, as well as many of the microbes themselves. The technology behind ATP systems has been changing quite rapidly over the past few years and now there are ATP systems that can run tests specifically for microorganisms.
For more information on these multi-functional ATP systems see the MicroSnap platform from Hygiena.
What is a biofilm?
A biofilm is formed when microorganisms find a receptive environment where they are exposed to food and moisture. The microorganisms work together as a population and secrete a sticky polymer to form a solid matrix attached to a surface. Once a biofilm is established, it is very difficult to eliminate because the microbes are reinforced and protected by the matrix, making them very resistant to sanitizers. Biofilms are often responsible for poor product quality and/or lost product due to contamination, causing costly damage to both product and equipment. The threat of a biofilm can be eliminated with proper ATP hygiene monitoring, allowing early detection and removal of food residue – thus eliminating the food source for possible biofilm-forming microbes. In addition, Hygiena ATP swabs have a unique detergent on the swab tip that cuts through biofilm and exposes the underlying cells. If a biofilm has already developed, there will be more ATP on a surface, which will result in a higher RLU result.

When should I do ATP testing?
ATP testing should ideally be done after cleaning, but before sanitization. Because sanitizers are less effective when product residues are on the surface, it’s best to eliminate all ATP before the sanitization step. In some facilities, testing after cleaning is not possible. In these scenarios testing after sanitization is acceptable. Most sanitizers will not affect Hygiena’s ATP test devices and if the proper concentration and dwell time is followed there should be no interference from sanitizers.

What is the difference between cleaning, disinfection, and sterilization?
Cleaning is the removal of organic matter and the reduction of risk from material which is a potential contaminant, or material which could support the survival and growth of microbes.
Disinfection is the reduction of microbiological hazards to a minimal level but not necessarily complete inactivation of all microbiological hazards.
Sterilization is the complete destruction and inactivation of all microbial hazard.
What areas should be tested?
Food contact areas (direct and indirect) and hard-to-clean areas should be the main focus of your swabbing program. Direct contact areas are surfaces where the presence of any contaminant will taint the final product. Indirect contact areas are those where splashed product, dust, or liquid has the potential to be dropped, drained, or transferred onto the product. Hard-to-clean areas may include filler heads, O-rings, nozzles, and areas with irregularly-shaped surfaces, corners, grooves and cracks.
What is the proper swabbing technique?
You should swab an area that is about 4 by 4 square inches (10 x 10 cm) – or in the case of a hard-to-clean area, as much of the surface as possible. Do not let the swab come into contact with anything other than the test area to avoid contamination. Apply pressure to the swab to pick up surface residue and penetrate any biofilm that may be present. After collecting the sample, place the swab back in swab tube. Once the device has been activated, it should be read as soon as possible
| Correct | Incorrect |
 |  |
Sufficient pressure to create flex in Do not touch the swab shaft Rotate to collect sample on all sides | Only lightly swabbing the surface Touching the swab shaft with finger Only collecting sample on one side |
How long are ATP tests good for?
ATP test devices have a 15 month shelf life at refrigerated temperatures (2-8° C) and 4 week shelf life at room temperature (21-25° C). All swab labels are printed with expiration month and year for your convenience.
How long can a test that has been sampled, but not activated by breaking the Snap Valve, be held before testing in the instrument?
Longer times between sample swabbing and luminometer testing resulted in significant reductions in RLUs, and raise the risk of false negative results. We recommend activation of test devices be conducted as soon as possible and no longer than 30 minutes after sample collection. Read the Tech Bulletin here.
Does a surface have to be dry before I can swab?
Surfaces do not have to be dry to perform a swab test. However, for consistent readings, surfaces should be swabbed in the same conditions (always wet or always dry). Hygiena swabs come pre-moistened for maximum sample recovery on dry surfaces.
What are Hygiena ATP swabs pre-moistened with? Is it safe?
Hygiena ATP test swabs are pre-moistened with a mild extraction solution that aids in sample collection and ATP extraction. The solutions is non-toxic and is not a growth media, so it is completely safe. Please refer to the Safety Data Sheet for the product for further information about safety.
How do I properly dispose of UltraSnap or SuperSnap?
Hygiena ATP test devices are made of 100% recyclable, non-toxic plastic and may be discarded with plastic recyclables.(Recycling Code 7:Other).
How do sanitizers or soap affect Hygiena products?
Several sanitizers commonly used in the food and beverage industry have been tested at normal working strength and found to have no significant effect on either Snapshot or UltraSnap performance. Only acid-based sanitizer, if used at higher than normal concentrations, have been found to have an effect on performance. If you are unsure about the chemical nature of your sanitizers, consult your sanitizer manufacturer or Hygiena technical support.
Will direct sunlight affect test results?
Yes. Tests activated and measured in direct sunlight will distort results, increasing the RLU output. If a test must be collected outside, the swab device should be activated and measured out of the direct sunlight.
How does temperature affect ATP test result?
Ambient temperature of 20–22°C (70–72°F) is the temperature that provides optimal performance. The only time ambient temperatures can affect results is if reagents are at a low temperature. This can happen if testing is done immediately after taking the tests out of the refrigerator. If testing in cold environments the instrument will self-adjust to external temperature but the luciferase reagents needs to be at 20–22°C (70–72°F) to function best.
If reagents are cold, then the reaction will be slower and the RLU result for a given amount of ATP will be lower.
Temperature does not affect light measurement of the instrument provided that the instrument has equilibrated to the environmental conditions. The instrument will sense an environmental temperature change of 5°C and it will automatically initiate a 15 second re-calibration sequence.
The user can also initiate a manual re-calibration at any time by pressing and holding the OK button down for 3 seconds.
The system can be used in cold environments (e.g. 5°C) if the instrument is equilibrated for 10 – 15 minutes before use and that the reagent swab devices are kept warm e.g. by storage in an internal pocket close to body heat.
Why do I get a different RLU result when I re-measure the same test device again in the luminometer?
You will always get a different reading when trying to re-read the same test device more than once. The meter is reading a light given off by the reagent. This light peaks and then begins to die off. Always measure your test device in the time recommended in the product instructions. Any result you receive after the first reading may be misleading because that light will continue to die out until eventually no light is emitted.
How do I use ATP testing, protein testing, and specific allergen testing together in my environmental allergen control program?
Incorporating high-sensitivity ATP testing (EnSURE & SuperSnap), high-sensitivity protein testing (AllerSnap), and specific surface allergen tests (AllerFlow Gluten) into your environmental monitoring program is a proactive and holistic way to prevent allergen contamination and validate and verify cleaning procedures. Hygiena has several documents available on how to use these products together. Download any of the resources listed here:
end faq
SnapShot, WaterShot
Do I have to change my RLU limits to use SnapShot or WaterShot?
No. SnapShot and WaterShot work just like the device they are replacing, so you may continue testing the same way. However, superior design and reagent technology make SnapShot and WaterShot more consistent, reliable, and affordable. Since each manufacturer's test is a little different, we have made a unique version of SnapShot for each of the systems out there. To understand how we did this, it is helpful to understand how ATP bioluminescence reaction works. The graph below shows that as the amount of ATP goes up, so does RLU. It is a linear reaction on all instruments.

The next graph shows how SnapShot gives the same readings as the one it is replacing. Different dilutions of ATP were pipetted onto an ATP test. The results are plotted on a line. Then the slope of the line is determined. SnapShot is then designed to have the same results and slope.

Why is SnapShot so much less expensive that the test I currently use?
Snapshot's design and Hygiena's manufacturing expertise allows us to charge less for SnapShot. In addition, the patented design also gives some added benefits. To better understand how the Snapshot design equates to greater cost savings, let us compare the BioControl's MVP™ and 3M's CleanTrace™ test to Hygiena's SnapShot. SnapShot uses 50% less plastic than other ATP tests and the Snap-Valve design eliminates the need for additional compartments that require foil seals. This reduces raw costs, production costs and is more environmentally friendly.

Are there any studies or white papers proving SnapShot works better than manufacturers swabs?
A leading international food testing and safety laboratory conducted an independent study comparing five commercial ATP systems. The study incorporated SnapShot with other manufacturer systems and found that Hygiena’s products offer superior linearity, sensitivity, repeatability, and accuracy. For more information, or a copy of the full report, click here.
How does SnapShot give more reproducible results?
There are two reasons that SnapShot gives more reproducible results:
1. SnapShot uses Hygiena's unique liquid-stable reagent. By formulating the luciferin/luciferase and buffer together in a liquid-stable format, we are able to eliminate additional steps that add variability such as freeze-drying of enzymes and a reconstitution step when the test is activated. The histograms below show the percent variation of 60 tests with the same ATP concentration. Tests were run on two systems and then run with SnapShot.

2. The patented design of SnapShot allows the luciferin/luciferase enzyme, buffer, and contamination collected by the swab bud to mix without having to puncture foil-sealed chambers that may cause loss of sample. The diagram below shows the process that takes place when a test device with foil seals compared to SnapShot, without foil seals.


How do I evaluate SnapShot against the current ATP device I am using?
Hygiena provides Side by Side evaluation kits that allow you to compare ATP test devices in a controlled environment. Doing a side by side comparison with this kit should not take more than 30 minutes. To request a Side by Side evaluation kit, contact Hygiena or a qualified distributor and request one of the following kits:
- SBS-SBC1575 - BioControl
- SBS-SPXL1333 - 3M/Biotrace
Hygiena makes SnapShot test for the following luminometers.
Luminometers -Catalog numbers:
- BioControl
- Lightning MVP - SBC1575 BioControl Lightning MVP virtual demo
- Lightning - SBC1575 BioControl Lightning virtual demo
- 3M / Biotrace
- Uni-Lite® NG - SPXL1333 3M / Biotrace NG virtual demo
- Uni-Lite® XCEL - SPXL1333 3M / Biotrace XCEL virtual demo
- Uni-Lite® - SPXL1333
- Uni-Lite® NG Junior - SPXL2020
- Merck
- Celsis
- Lumac M™ - SS1211
- SystemSURE - SS1211
- For Kikkoman PD-10 luminometers, see UltraSnap US2020
What is the proper swabbing technique with SnapShot swabs?
You should swab an area that is about 4 by 4 square inches (10 x 10 cm) – or in the case of a hard-to-clean area, as much of the surface as possible. Do not let the swab come into contact with anything other than the test area to avoid contamination. Apply pressure to the swab to pick up surface residue and penetrate any biofilm that may be present. After collecting the sample, place the swab back in swab tube. Once the device has been activated, it should be read as soon as possible.
| Correct | Incorrect |
 |  |
Sufficient pressure to create flex in Do not touch the swab shaft Rotate to collect sample on all sides | Only lightly swabbing the surface Touching the swab shaft with finger Only collecting sample on one side |
How long are SnapShot and WaterShot tests good for?
ATP test devices have a 15 month shelf life at refrigerated temperatures (2-8° C) and 4 week shelf life at room temperature (21-25° C). All swab labels are printed with expiration month and year for your convenience.
How do I properly dispose of SnapShot?
SnapShot is made of 100% recyclable, non-toxic plastic and may be discarded with plastic recyclables.(Recycling Code 7:Other).
Does a surface have to be dry before I can swab?
Surfaces do not have to be dry to perform a swab test. However, for consistent readings, surfaces should be swabbed in the same conditions (always wet or always dry). Hygiena swabs come premoistened for maximum sample recovery on dry surfaces.
How do sanitizers or soap affect Hygiena products?
Several sanitizers commonly used in the food and beverage industry have been tested at normal working strength and found to have no significant effect on either Snapshot or UltraSnap performance. Only acid-based sanitizer, if used at higher than normal concentrations, have been found to have an effect on performance. If you are unsure about the chemical nature of your sanitizers, consult your sanitizer manufacturer or Hygiena technical support.
Will direct sunlight affect test results?
Yes. Tests activated and measured in direct sunlight will distort results, increasing the RLU output. If a test must be collected outside, the swab device should be activated and measured out of the direct sunlight.
How does temperature affect ATP test result?
Ambient temperature of 20–22°C (70–72°F) is the temperature that provides optimal performance. The only time ambient temperatures can affect results is if reagents are at a low temperature. This can happen if testing is done immediately after taking the tests out of the refrigerator. If testing in cold environments the instrument will self-adjust to external temperature but the luciferase reagents needs to be at 20–22°C (70–72°F) to function best.
If reagents are cold, then the reaction will be slower and the RLU result for a given amount of ATP will be lower.
Temperature does not affect light measurement of the instrument provided that the instrument has equilibrated to the environmental conditions. The instrument will sense an environmental temperature change of 5°C and it will automatically initiate a 15 second recalibration sequence.
The user can also initiate a manual recalibration at any time by depressing and holding the OK button down for 3 seconds.
The system can be used in cold environments (e.g. 5°C) if the instrument is equilibrated for 10 – 15 minutes before use and that the reagent swab devices are kept warm e.g. by storage in an internal pocket close to body heat.
Why do I get a different RLU result when I re-measure the same test device again in the luminometer?
You will always get a different reading when trying to re-read the same test device more than once. The meter is reading a light given off by the reagent. This light peaks and then begins to die off. Always measure your test device in the time recommended in the product instructions. Any result you receive after the first reading may be misleading because that light will continue to die out until eventually no light is emitted.
end faq
Monitoring Systems
EnSURE Touch, EnSURE, SystemSURE Plus
Does the system need to be calibrated?
Hygiena ATP systems self-calibrate at start up to ensure accurate readings. To further verify calibration, we offer a calibration control kit. Though the kit is not necessary for the performance of the system, a documented calibration process shows due diligence to auditors. With advanced technology, Hygiena systems will stay in calibration for the life of the device. If you require a manufacturer's calibration as part of your quality program, you can request a quote for calibration here.
Are there maintenance costs I should be worried about?
Unlike other systems on the market, Hygiena’s EnSURE and SystemSURE Plus do not require expensive yearly maintenance or service contracts. Monitoring systems come with a 1 year warranty. Should you system become damaged or non-operational, Hygiena will promptly replace your device with a loaner so that you do not experience any interruption in your testing program.
Does the system do anything else besides ATP testing?
In addition to surface and water ATP testing, Hygiena’s EnSURE measures test swabs for:
How do I erase the results off the handheld?
There are two ways to erase the results off the handheld.
- If you are using SureTrend software, simply plug connect your luminometer to your computer, click "Synchronize" in SureTrend, upload results, and click "Yes" when asked if you would like to erase test results memory.
- If you want to erase the results without syncing to SureTrend, select the * button on the meter, then use the arrows to navigate to the Memory menu. Press "OK" to select, and then press and hold down the * button for 2 seconds. This will display the total number of stored results to be erased. To accept and start the erase function, press and hold down the "OK" button for 1 second. To cancel, press any other button.
Why do EnSURE and SystemSURE Plus come pre-programmed with Pass/Fail limits of 10 and 30?
The pre-programmed limits for food and beverage facilities are based on years of data, experience, and third part studies. Hygiena recommends that users validate these recommendations and adjust them to meet each facility's unique needs. To see the data or learn more, download this ATP Thresholds Technical Document.
I'm getting higher than expected results. What is wrong?
- Results are relatively unaffected by the majority of sanitizers evaluated, which includes sanitizers incorporating a wide variety of active ingredients. Under certain conditions, some sanitizers may give a slight increase in readings. Read full Technical Document
- You may need to adjust your thresholds to the type of surface or environment that you are testing. Certain environments can offer higher than average results. Read full Technical Document
- If these tips do not resolve the problem, contact technical support.
I'm getting lower than expected results. What is wrong?
Almost all unexpected results can be resolved by asking yourself these questions:
- Have my swabs been stored at the correct temperatures and are within the expiration date? Swabs that have been stored incorrectly for prolonged periods of time may not perform as expected.
- Am I holding the meter upright when taking measurement? Holding the meter upright ensures that the sensor at the bottom of the machine can see and quantify the light reaction accurately.
- Am I overloading the swab? Swabbing too large of an area, or a physically soiled surface, may result in picking up physical debris. This debris will inhibit the light reaction, giving lower than expected results.
- Is the read chamber dirty? A dirty read chamber could also block light from reaching the sensor. To check your read chamber, open the lid of your system, and use the vertical plastic tab to pull the read chamber out of the meter. (You may also find that pushing your finger into the chamber then lifting it out works easier for you.) If you see any water spots or debris on the clear plastic tip of the read chamber, you may wash the read chamber with warm water and a very mild detergent and then let it air dry. Ensure the chamber is completely dry before replacing in the meter. If the read chamber has physical damage like cracks or scratches on the clear plastic tip, contact customer service to order a replacement read chamber.
If these tips do not resolve the problem, contact technical support.
Why do I sometimes get 1-4 RLU with the negative control in the Calibration Control Kit?
Though very rare, there are a few conditions under which Hygiena luminometers can occasionally measure very low RLU levels. Most commonly, this is due to debris on the swab or in the read chamber, or when swabs or controls are exposed to bright light for extended periods of time just prior to use. The read chamber can be removed and hand-washed and you can refer to product instructions for proper swab storage. Another uncommon scenario which may cause an unexpected RLU reading on negative samples is when there is a static charge on the swab device before insertion in the luminometer. If you experience unexpected RLU measurements, contact a technical representative for help troubleshooting.
I accidentally placed a wet swab into the read chamber. How can I clean the instrument?
One of the great features of Hygiena's EnSURE and SystemSURE Plus is the removable read chamber that can be hand washed in the event of situations like these. To remove the read chamber, open the lid of the system, and insert your finger into the read chamber, and pull it out. The read chamber can be washed with warm soapy water and allowed to air dry. Once completely dry, the read chamber may be replaced in the system. See more info about the removable read chamber and other convenient features here.
Why are Hygiena's products so much more cost effective?
Hygiena strives to create easier-to-use, more cost-effective products for its clients. By partnering with leading manufacturing companies and using state-of-the-art automated manufacturing processes in house, Hygiena is able to create superior cost-effective products. A perfect example of this is our ATP bioluminescence product line. It incorporates Medical Packaging's patented user-friendly and environmentally-friendly Snap-Valve™ technology, which allows us to create an easy-to-use sample test device that uses less plastic than our competition and has superior performance. Combining this with our unique liquid-stable reagents allows us to have a complete all-in-one sample testing device that has better performance and costs a lot less.
How do I contact Hygiena to purchase an ATP solution?
Hygiena products are available worldwide. We have a direct sales staff in the USA, UK and parts of Europe, and Asia. Fill out a contact form here or call us at the phone number at the top right of this web page. Hygiena also has a network of dedicated distributors and agents across the globe, covering over 80 countries. Hygiena's sales team and distributors have specific skills and experience in providing products in the food processing market, particularly ATP bioluminescence and its applications. To find a distributor in a particular part of the world, click here.
end faq
SureTrend Software
Where can I download SureTrend software?
Go here: SureTrend Software Download Information
I have an old version of SureTrend software (3.0 or earlier). How can I update to the latest version?
Find details here: SureTrend Software Download Information
Do I need to dedicate a database server to use SureTrend Version 4.0?
SureTrend Version 4.0 uses Microsoft SQL Server to store its data. By default SureTrend will install and use the Microsoft SQL Compact 3.5 edition for single and multi-user installations. You can upgrade the database to SQL Server Express and above, and then configure the SureTrend client to use your SQL server.
How do I install SureTrend on a network server?
For SureTrend 3, follow the instructions in this document: How to Install SureTrend on a Network . For newer versions, see specifications here: SureTrend Software Download Information. If you run into any trouble, chat with us via live chat, or contact tech support by filling out this form.
Why is SureTrend showing me an error that says "The model type is different..." ?
Newer models of EnSURE or SystemSURE Plus (with a V2 next to the serial number) are not compatible with SureTrend versions 2.03 or earlier. The expanded storage capacity of V2 luminometer models prevents earlier versions of SureTrend from syncing correctly with the luminometer. All you have to do is upgrade your version of SureTrend! Follow this easy step by step guide: Upgrading to SureTrend Version 3.01 . Please feel free to contact Hygiena technical support for assistance.
What OS is recommended for Hygiena's SureTrend Software?
See specifications here: SureTrend Software Download Information
Can I run SureTrend on a tablet?
Yes, as long as the tablet meets the hardware and software requirements listed above in "What OS is recommended for Hygiena's SureTrend Software?".
IMPORTANT: Windows RT tablets are not supported.
Is there a version of SureTrend for Mac?
No, SureTrend was designed for Windows, but you can run SureTrend on a Mac using software like Parallels or other virtualization software. The important requirement for the virtual machine is USB support. Some older versions of Parallels, VMWare, Microsoft Virtual PC do not support USB, so it is important to have a current version. If SureTrend is installed on a terminal server or if the user is remoting to the virtual machine, there may be issues with USB redirection.
Does SureTrend support Virtual Desktop or RDP?
SureTrend can run on a Windows Terminal Server (RDS), Citrix, or other VNC software that will remote the COM port from the server to the desktop. Since SureTrend is running on the server, but the unit is connected to the desktop COM port, communication to the Unit is directed over the network. If your network latency is too high you may get COM port communication failures that require you to repeat the synchronization process with the Unit. Also, the SureTrend feature that automatically detects the COM port will not work and user must know the COM port used on the desktop.
Does SureTrend support virtualization such as Windows XP mode or VMWare?
SureTrend will run on virtualized version of Windows supported by SureTrend as long as the virtualization software supports COM ports or USB. Some users are running Windows 7 or 8 on their desktop computer, but spend most of their time in a Windows XP mode session to support other legacy applications in their organization. Having SureTrend run in the Windows XP mode helps the user stay in the Windows XP mode and not have to switch to the native desktop just to run SureTrend.
end faq
DNA-Based Pathogen Detection
BAX® System
What's the difference between real-time and standard PCR assays?
The BAX® System PCR tablets in standard assays contain fluorescent dye, which binds with double-stranded DNA and emits a fluorescent signal in response to light. After amplification, the BAX® System begins a detection phase where the fluorescent signal is measured. During detection, the temperature of the samples is raised to the point where the DNA strands separate (denature), releasing the dye and lowering the signal. This change in fluorescence can be plotted against temperature to generate a melting curve, which is interpreted by the BAX® System software as positive or negative results.
The BAX® System PCR tablets used in real-time assays contain multiple target-specific, dye labeled probes. Probes are short oligonucleotides with quencher dye at one end that greatly reduces fluorescence from the fluorophore dye at the opposite end. During PCR, probes bind to a specific area within the targeted fragment and the fluorophore is separated from the quencher, allowing for increased fluorescent signal. The BAX® System Q7 instrument uses dye-specific filters to measure signal at the end of each cycle and report positive/negative results for each target. In general, real-time assays offer enhance specificity, sensivity and time-to-result over standard assays.
What are the main differences between the BAX® System Q7 and BAX® System X5 instruments?
The main differences between the two BAX® System instruments are cost, size and throughput. Additionally, while the BAX® System Q7 instrument is able to process all targets in our portfolio, the BAX® System X5 focuses on the core pathogens of interest such as pathogenic E. coli, Salmonella, and Listeria.
What total time to result does the BAX® System provide?
The BAX® System can provide results in as little as 90 minutes for isolated colonies and 51 hours for the more traditional methods. A majority of methods have been optimized to provide next day results.
Is it possible to use commercially available media instead of BAX® System enrichment media?
Yes, there are various protocols that allow non-proprietary media to be used.
Can multiple pathogens be detected in one well with the BAX® System?
No, different pathogens (i.e. Salmonella and Listeria) cannot be detected in the same well. However, protocols exist that allow you to use a single enrichment to grow multiple targets, which reduces the number of enrichments and downstream consumables and step required. Additionally, select assays (Campylobacter, Vibrio, and STEC) will detect multiple pathogenic strains of said pathogens in a single well reaction well.
I have an ABI 7500 FAST instrument; can I use it to run BAX® System assays?
Yes. With the purchase of a BAX® System Q7 work station conversion package you can operate the ABI 7500 fast instrument as a BAX® System Q7.
Are the BAX® System's software results given as only +/- , or is it possible to estimate the number of CFU?
The BAX® System Q7 is capable of “real-time” PCR whereby target amplification and detection take place simultaneously. Amplification plots are displayed after analysis that represent the accumulation of florescent signal measured (DNA) with respect to PCR cycle. One can estimate the level of target in the reaction by assessing the “Cycle Threshold” (Ct) value, however studies have demonstrated flaws with this approach as it relates to estimating concentrations of live, healthy cells pre and post enrichment.
For many BAX® System assays, threshold protocols exist whereby samples containing target bacteria below a given level are considered negative, and only samples above that level are considered positive. The protocols are dependent on the initial concentration of target in a sample, the amount of homogenized sample enriched, and the level of confidence one is willing sacrifice based on probability theory.
The BAX® System has also been used in a Most Probable Number (MPN) format to estimate target density in an initial sample. In this protocol, a sample is homogenized and aliquots of the homogenate are dispensed into enrichment tubes. Once incubated, the tubes can be processed on the BAX® System, and the number of tubes determined to be positive can be used to calculate the most probable number of organisms in the original sample.
Does the BAX® System Q7 come with protocols specifying how to collect the sample?
No, the system does not come with specific protocols for sample collection that you must follow. When collecting samples, we recommend that you follow your internal standard operating procedures (consult your regional governing bodies and/or industry associations for accepted sampling practices).
end faq
BAX® System PCR Assay for Salmonella 2
What is the difference between the original Salmonella assay and Salmonella 2?
The PCR tablets in the Salmonella 2 assay contain a new "hot-start" technology. This proprietary technology provides a more robust assay by preventing the reaction enzyme from activating until PCR begins. This greatly reduces the opportunity for non-specific PCR product to form and improves specificity.
Why is this change being made?
As part of Hygiena's commitment to continuous improvement, we saw an opportunity to incorporate a new technology that was not available when the Salmonella kit was first introduced in 1995. Analysis of the original Salmonella assay suggested that atypical results could occur due to the formation of non-specific product before PCR begins. This can happen when there is a delay between hydrating the PCR tablets and starting the PCR reaction. Sometimes the delay is due to user distraction; other times, it can be due to the very brief lag time in BAX® System Q7 instrument as the rack rises into position.
Why is this change being made specifically to the Salmonella assay?
We initiated this improvement with the Salmonella assay because Salmonella, more so than other pathogens, has close genetic neighbors that can generate non-specific product. Also, as one of the top foodborne pathogens, Salmonella testing will have the greatest impact on food safety.
Will this improvement be applied to other assays as well?
BAX® System real-time assays already incorporate ingredients for hot-start effect. We will evaluate the need for incorporating this improvement into other end-point assays on an individual basis.
Do these tablets use a commercial hot-start technology?
No. The hot-start effect of the Salmonella 2 tablets is due to a proprietary technology.
Why not use a commercial hot-start polymerase instead?
Internal studies have shown three major advantages to the proprietary technology:
- The proprietary technology is more effective for eliminating non-specific product than a commercial hot-start polymerase.
- The proprietary technology is more broadly applicable, which will allow us to incorporate this improvement to new products in the future.
- This technology is more cost effective, which helps to keep the price of the Salmonella 2 kit to the same list price as the original kit.
Do I need to re-calibrate my BAX® System Q7 instrument before running this assay?
No. The Salmonella 2 assay uses the same dye as the original Salmonella assay, so no recalibration is necessary.
Do I have to do anything differently to run the Salmonella 2 assay?
No. The Salmonella 2 assay follows the same enrichment, sample prep and processing protocols as the original Salmonella assay.
What do the Salmonella 2 results look like?
Melt curves are the same as those of the original Salmonella assay, with three target peaks in the range of 84°C - 92°C.
Can the Salmonella 2 assay be run with other BAX® System assays?
The Salmonella 2 assay can be run with any standard (not real-time or reverse-transcriptase) assay. For validation purposes, both Salmonella assays can be run together (The original assay has a solid black stripe on the edge of the strip of tubes; the Salmonella 2 strips do not have any stripes).
Is the Salmonella 2 assay a validated method?
Yes, the Salmonella 2 assay has been submitted to AOAC and AFNOR and approved as a modification to the original Salmonella assay approved method.
end faq
StatMedia™ Soluble Packets
What are Hygiena™ StatMedia™ Soluble Packets?
Hygiena™ StatMedia™ soluble packets provide a new delivery system for enrichment media. They are unit-dose packets of powdered media in a water-soluble film.
What are the advantages of using Hygiena™ StatMedia™ Soluble Packets?
Hygiena™ StatMedia™ soluble packets are unit-dose packets of powdered enrichment media in a water-soluble film. The convenience of these pre-weighed packets simplifies and shortens the media preparation process, eliminates dust exposure and reduces media preparation costs, with less waste from expired rehydrated media. It also reduces dependency on autoclaving, along with those associated costs.
How do I use Hygiena™ StatMedia™ Soluble Packets?
Simply drop a soluble packet into a steril container with the appropriate volume of pre-warmed, sterilized water. The water-soluble film dissolves within secondsm, and with gentle mixing the media dissolves fully.
Does the water-soluble film affect the rehydrated media?
No. Internal studies show that the film does not interfere with bacterial growth, lysis or PCR sensitivity.
Do I have to autoclave the media after it is prepared?
Media prepared with Hygiena™ StatMedia™ soluble packets does not need to be autoclaved if it is used within 4 hours.
How long can I store prepared media after it is autoclaved?
Prepared, autoclaved broth can be stored away from light for up to 2 weeks at 2-8°C. Before using, make sure to pre-warm the broth to 42°C.
How can I store the StatMedia™ Soluble Packets?
Cartons and foil pouches can be stored at room temperature (10-25°C) and used for up to one year from the manufacturing date stamped on the pouch.
What should I use to handle the media packets?
Use either sterile gloves or tongs to remove the packets from pouches. Doing so lessens the possibility of contaminating other packets in the pouch or the packet in use. After removing a packet, always re-seal the foil pouch strip.
Why is it important to keep the foil pouch sealed?
The re-sealable foil pouch prevents the packets from absorbing water from either a spill or humidity in the ambient room air.
What happens if the packets get wet while in the pouch?
If a pouch is not sealed properly, and a packet absorbs water, the media inside the packet may become discolored and granular. In addition, the water soluble film may show evidence of localized or dispersed areas where the film has begun to dissolve. If either occurs, the packet should be discarded according to your facility’s procedures.
end faq
24LEB Buffer Supplement
What is 24 LEB Buffer Supplement?
The 24 LEB Buffer Supplement is a reagent that can be added to 24 LEB enrichment broth to maintain the pH level for certain food types when testing with the BAX® System 24E assays.
Why would I use 24 LEB Buffer Supplement?
Some food types have naturally low pH levels or may experience a drop in pH during enrichment in 24 LEB. These lower pH levels can affect the performance of the BAX® System, sometimes causing inaccurate results. AFNOR studies validated that adding 24 LEB Buffer Supplement to the enrichment media maintains the pH level for food types at risk of experiencing a drop in pH during enrichment. Note: Low pH levels are only one factor that may contribute to poor Listeria growth in sample enrichments. This supplement has not been validated to improve performance in foods that naturally inhibit the growth of Listeria for reasons other than low pH levels.
Is this additional reagent part of the AOAC-approved protocol?
No, the foods validated by AOAC did not require use of the 24 LEB Buffer Supplement.
How do I internally validate my sample type?
Before testing any food types that have not been certified by AFNOR or AOAC, it is strongly recommended that you internally validate samples with the assay to determine if the buffer supplement is required. You can evaluate your sample type to determine if the 24 LEB Buffer Supplement is necessary by following these steps.
1. Prepare 24 LEB enrichment broth by combining 24 LEB base and selective supplement as described in the BAX® System User Guide or package insert.
2. Stomach sample in 1:10 ratio with room-temperature prepared 24 LEB enrichment broth.
3. Use a pH strip to determine the pH of the homogenized sample.
a. If the pH is lower than 6.5, then the 24 LEB buffer supplement should be used with the sample type.
b. If the pH is higher than 6.5, continue with step 4.
4. Incubate sample at 37°C for 24-28 hours, then re-check the pH of the sample.
a. If the pH is lower than 6.0, then the 24 LEB buffer supplement should be used with the sample type.
b. If the pH is higher than 6.0, then the 24 LEB buffer supplement is not necessary for the sample type.
end faq
Hygiena™ Lateral Flow System™ for E. coli O157
What is the sensitivity of the Hygiena Lateral Flow System?
it detects 1 cfu/25g sample.
Which species does LFS for E. coli O157 detect?
The test is specific for all types of E. coli O157, including O157:H7, O157:Non motile and other O157:H factors.
If I use a screening test for O157, am I complying with the USDA-FSIS regulations?
Yes, USDA-FSIS 10.010 states that a sample is considered positive if it is (a) positive for O157 and H7, or (b) positive for O157, and it is non-motile or the H factor cannot be determined, and it is positive for one or both of the shiga toxins.
How has the test been validated?
Samples of this test kit model were independently evaluated by the AOAC Research Institute and were found to perform to the producer's specifications as stated in the test kit's descriptive insert. The producer certifies this kit conforms in all respects to the specifications originally evaluated by the AOAC Research Institute as detailed in the Performance Tested Certificate number 010601.
Which foods have been tested?
Raw boneless beef, ground beef and apple cider have been tested. In addition, field testing has been performed by several customers on composite beef samples (375g), raw and cooked chicken, carcass swabs, mushrooms, and dairy products.
Does the test cross-react with other Enterobacteriacae or any other organism that may be present in the food sample?
To our knowledge, the test does not react with any bacteria other than E. coli O157. A number of customers have sent in samples that have been difficult to culture or identify on culture media and all of these samples were found to be E. coli O157.
end faq
Microorganism Tests
MicroSnap Total
How fast does MicroSnap Total deliver results?
MicroSnap Total delivers results in 7 hours. This time frame reflects the entire test time, including the enrichment and detection steps.
Does MicroSnap Total detect both aerobic and anaerobic bacteria?
MicroSnap Total is a rapid method for obtaining a total viable count (TVC) of the bacteria present in a sample. It will detect all bacteria that are capable of aerobic respiration, e.g., facultative anaerobes (bacteria that can use oxygen if present or use fermentation for energy in the absence of oxygen), but it will not detect obligate anaerobes (bacteria that will not grow in the presence of oxygen).
Is it possible to collect my sample ahead of time and run the assay later? For example, I want to collect samples at the end of a shift, and then conduct the test the following day.
Yes. If you are testing for qualitative results (presence/absence), then it is possible to collect a sample in advance and delay the assay. To do this, collect the sample according to the instructions and put the entire unactivated enrichment device in a refrigerator (2-8C). When you are ready to begin the test process, remove the enrichment device from the refrigerator and activate the device according to instructions. Advance sample collection for enumeration testing has not been thoroughly tested by Hygiena laboratories and is not recommended.
What happens if I incubate the enrichment device for longer than the 7 hours directed?
If you are looking for presence/absence results, additional incubation will only heighten test sensitivity, so extended incubation is not harmful. However, if you are looking for enumeration results, only incubate the enrichment device for the directed 7 hours.
Why do I get a different RLU result when I re-measure the same test device again in the luminometer?
You will always get a different reading when trying to re-read the same test device more than once. The meter is reading a light given off by the reagent. This light peaks and then begins to die off. Always measure your test device in the time recommended in the product instructions. Any result you receive after the first reading may be misleading because that light will continue to die out until eventually no light is emitted.
What is the swabbing solution on MicroSnap enrichment device swabs?
The swab wetting solution used in MicroSnap is a standard sample collection liquid used commonly in food microbiology applications. It is based on Butterfields buffer.
end faq
MicroSnap EB (Enterobacteriaceae)
How fast does MicroSnap EB deliver results?
MicroSnap EB delivers results in 6 to 8 hours depending on the sensitivity required. This time frame reflects the entire test time, including the enrichment and detection steps.
Is it possible to collect my sample ahead of time and run the assay later? For example, I want to collect samples at the end of a shift, and then conduct the test the following day.
Yes. If you are testing for qualitative results (presence/absence), then it is possible to collect a sample in advance and delay the assay. To do this, collect the sample according to the instructions and put the entire unactivated enrichment device in a refrigerator (2-8C). When you are ready to begin the test process, remove the enrichment device from the refrigerator and activate the device according to instructions. Advance sample collection for enumeration testing has not been thoroughly tested by Hygiena laboratories and is not recommended.
What happens if I incubate the enrichment device for longer than the 8 hours directed?
If you are looking for presence/absence results, additional incubation will only heighten test sensitivity, so extended incubation is not harmful. However, if you are looking for enumeration results, only incubate the enrichment device for the directed time.
Why do I get a different RLU result when I re-measure the same test device again in the luminometer?
You will always get a different reading when trying to re-read the same test device more than once. The meter is reading a light given off by the reagent. This light peaks and then begins to die off. Always measure your test device in the time recommended in the product instructions. Any result you receive after the first reading may be misleading because that light will continue to die out until eventually no light is emitted.
What is the swabbing solution on MicroSnap enrichment device swabs?
The swab wetting solution used in MicroSnap is a standard sample collection liquid used commonly in food microbiology applications. It is based on Butterfields buffer.
end faq
MicroSnap Coliform
How fast does Microsnap Coliform deliver results?
MicroSnap Coliform delivers results in 6-8 hours from sample collection to results. Enumeration or quantitative results are available in 6 hours. Presence/absence or qualitative results are available in 8 hours. These time frames reflect the entire test time, including the enrichment and detection steps.
What happens if I incubate the enrichment device for longer than the time in the directions?
If you are looking for presence/absence results, additional incubation will only heighten test sensitivity, so extended incubation is not harmful. However, if you are looking for enumeration results, only incubate the enrichment device for the directed 6 hours.
Is it possible to collect my sample ahead of time and run the assay later? For example, I want to collect samples at the end of a shift, and then conduct the test the following day.
Yes. If you are testing for qualitative results (presence/absence), then it is possible to collect a sample in advance and delay the assay. To do this, collect the sample according to instructions and put the entire unactivated enrichment device in a refrigerator (2-8C). When you are ready to begin the test process, remove the enrichment device from the refrigerator and activate the device according to instructions. Advance sample collection for enumeration testing has not been thoroughly tested by Hygiena laboratories and is not recommended at this time.
Why do I get a different RLU result when I re-measure the same test device again in the luminometer?
You will always get a different reading when trying to re-read the same test device more than once. The meter is reading a light given off by the reagent. This light peaks and then begins to die off. Always measure your test device in the time recommended in the product instructions. Any result you receive after the first reading may be misleading because that light will continue to die out until eventually no light is emitted.
Do MicroSnap Coliform or E. coli detect STECs such as E. coli O157?
No. Microsnap Coliform and E. coli detect general Coliforms and E. coli and not STECs such as O157.
What is the swabbing solution on MicroSnap enrichment device swabs?
The swab wetting solution used in MicroSnap is a standard sample collection liquid used commonly in food microbiology applications. It is based on Butterfields buffer.
end faq
MicroSnap E. coli
How fast does MicroSnap E. coli deliver results?
MicroSnap E. coli delivers results in 6-8 hours from sample collection to results. Enumeration or quantitative results are available in 6 hours. Presence/absence or qualitative results are available in 8 hours. This time frame reflects the entire test time, including the enrichment and detection steps.
What happens if I incubate the enrichment device for longer than the time in the directions?
If you are looking for presence/absence results, additional incubation will only heighten test sensitivity, so extended incubation is not harmful. However, if you are looking for enumeration results, only incubate the enrichment device for the directed 6 hours.
Is it possible to collect my sample ahead of time and run the assay later? For example, I want to collect samples at the end of a shift, and then conduct the test the following day.
Yes. If you are testing for qualitative results (presence/absence), then it is possible to collect a sample in advance and delay the assay. To do this, collect the sample according to instructions and put the entire unactivated enrichment device in a refrigerator (2-8C). When you are ready to begin the test process, remove the enrichment device from the refrigerator and activate the device according to instructions. Advance sample collection for enumeration testing has not been thoroughly tested by Hygiena laboratories and is not recommended.
Why do I get a different RLU result when I re-measure the same test device again in the luminometer?
You will always get a different reading when trying to re-read the same test device more than once. The meter is reading a light given off by the reagent. This light peaks and then begins to die off. Always measure your test device in the time recommended in the product instructions. Any result you receive after the first reading may be misleading because that light will continue to die out until eventually no light is emitted.
Do MicroSnap Coliform or E. coli detect STECs such as E. coli O157?
No. Microsnap Coliform and E. coli detect general Coliforms and E. coli and not STECs such as O157.
What is the swabbing solution on MicroSnap enrichment device swabs?
The swab wetting solution used in MicroSnap is a standard sample collection liquid used commonly in food microbiology applications. It is based on Butterfields buffer.
end faq
InSite Listeria
What strains of Listeria does InSite Listeria detect?
InSite detects Listeria monocytogenes, L. innocua, L. ivanovii, and L. welshmeri strains.
How do I dispose of InSite Listeria?
Autoclave or soak in bleach solution for 1 hour, then dispose of InSite in the trash.
I see black spots on the InSite Listeria swab bud. Is that positive?
Yellow/Amber media with black spots on the swab bud should be considered as a presumptive negative for Listeria spp.
How soon should InSite Listeria swabs be incubated after swabbing occurs?
Keeping samples at room temperature for hours/days at a time can promote growth of non-target organisms. The sponges on InSite Listeria do not have any antibiotics. This could potentially increase the likely hood of presumptive positives from non--target organisms.
The FDA Bacteriological Analytical Method for Salmonella and Listeria environmental sampling recommends testing your sample immediately (within an hour of collection). If this is not feasible, collected samples should be placed at refrigerated temperatures and tested within 48 hours. If samples are not going to be tested immediately after sampling, the recommendation is to store them at refrigerated temperatures until you are ready to test. If samples are to be stored in the fridge, we want to make sure the devices are brought up to room temperature before starting the incubation period. Drastic temperature changes from 4C to 37C could potentially harm the bacteria collected.
I see very small orange dots occasionally in the media. Is something wrong with the test?
A naturally occurring orange precipitation may be seen in some devices. This is due to excess iron falling out of solution. To alleviate this; the device can be shaken gently to dissolve, either before or after incubation, and used as normal. This will not affect the test result.
The media turned brown/black after incubating for longer than 48 hours, is this a presumptive positive?
All tests should be considered negative if the color of the media hasn't changed to brown/black after 48 hours. The media could turn brown/black after 48 hours without the presence of Listeria spp. present. This has to do with the antibiotics in the media being used up and other organisms overwhelming the chemistry. The media after 48 hours will pick up other organisms or fungi that produce the enzyme capable of producing the brown/black color.
How do I confirm presumptive positives?
Presumptive positive samples can be confirmed by streaking samples onto commonly used selective Listeria agar plates such as Modified Oxford Agar, Palcam agars or any recognized confirmatory procedure. Typical Listeria colonies on selective agar plates could then be further analyzed by more definitive tests such as microscopy, biochemical tests, etc. Some Enterococcus spp. are capable of giving false positive reactions with the test.
Are there any organisms that produce a false positive?
Some Enterococcus spp. are capable of giving false positive reactions with the test.
Is InSite Listeria AOAC approved?
InSite Listeria is not AOAC approved as a device. However, the broth in the device is AOAC approved.
What is the risk from the residual swabbing agent on InSite Listeria?
The swab wetting solution used in InSite Listeria is a standard sample collection liquid used in most food microbiology applications. It is based on Buffered Peptone Water that is used for the collection and recovery of microbes from product contact surfaces. The swab wetting solution is non-hazardous, sterile and does not contain any inhibitory substances. The swab wetting liquid contains a very small amount of protein (of non-bovine origin) that is buffered by inorganic phosphates.
end faq
InSite Salmonella
I want to activate InSite Salmonella immediately and skip the pre-enrichment step. How does this affect the test results?
Omitting pre-enrichment reduces the efficiency of Salmonella recovery and increases the probability of false negative results.
I want to extend the pre-enrichment time longer than the recommended 6 hours. How does this affect the test results?
Extending pre-enrichment beyond 6 hours increases the probability of false positive results.
How soon should InSite Salmonella
Allosteric sensitization of proapoptotic BAX
Abstract
BCL-2-associated X protein (BAX) is a critical apoptotic regulator that can be transformed from a cytosolic monomer into a lethal mitochondrial oligomer, yet drug strategies to modulate it are underdeveloped due to longstanding difficulties in conducting screens on this aggregation-prone protein. Here, we overcame prior challenges and performed an NMR-based fragment screen of full-length human BAX. We identified a compound that sensitizes BAX activation by binding to a pocket formed by the junction of the α3–α4 and α5–α6 hairpins. Biochemical and structural analyses revealed that the molecule sensitizes BAX by allosterically mobilizing the α1–α2 loop and BAX BH3 helix, two motifs implicated in the activation and oligomerization of BAX, respectively. By engaging a region of core hydrophobic interactions that otherwise preserve the BAX inactive state, the identified compound reveals fundamental mechanisms for conformational regulation of BAX and provides a new opportunity to reduce the apoptotic threshold for potential therapeutic benefit.
Access options
Subscribe to Journal
Get full journal access for 1 year
55,14 €
only 4,60 € per issue
All prices are NET prices.
VAT will be added later in the checkout.
Rent or Buy article
Get time limited or full article access on ReadCube.
from$8.99
All prices are NET prices.
Accession codes
Accessions
Protein Data Bank
References
- 1
Suzuki, M., Youle, R.J. & Tjandra, N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell103, 645–654 (2000).
CASPubMed Google Scholar
- 2
Edlich, F. et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell145, 104–116 (2011).
CASPubMedPubMed Central Google Scholar
- 3
Czabotar, P.E. et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell152, 519–531 (2013).
CASPubMed Google Scholar
- 4
Edwards, A.L. et al. Multimodal interaction with BCL-2 family proteins underlies the proapoptotic activity of PUMA BH3. Chem. Biol.20, 888–902 (2013).
CASPubMedPubMed Central Google Scholar
- 5
Gavathiotis, E., Reyna, D.E., Davis, M.L., Bird, G.H. & Walensky, L.D. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol. Cell40, 481–492 (2010).
CASPubMedPubMed Central Google Scholar
- 6
Gavathiotis, E. et al. BAX activation is initiated at a novel interaction site. Nature455, 1076–1081 (2008).
CASPubMedPubMed Central Google Scholar
- 7
Barclay, L.A. et al. Inhibition of pro-apoptotic BAX by a noncanonical interaction mechanism. Mol. Cell57, 873–886 (2015).
CASPubMedPubMed Central Google Scholar
- 8
Ma, J. et al. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc. Natl. Acad. Sci. USA109, 20901–20906 (2012).
CASPubMed Google Scholar
- 9
Petros, A.M. et al. Solution structure of the antiapoptotic protein bcl-2. Proc. Natl. Acad. Sci. USA98, 3012–3017 (2001).
CASPubMed Google Scholar
- 10
Walensky, L.D. & Gavathiotis, E. BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem. Sci.36, 642–652 (2011).
CASPubMedPubMed Central Google Scholar
- 11
Souers, A.J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med.19, 202–208 (2013).
CASPubMed Google Scholar
- 12
Sattler, M. et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science275, 983–986 (1997).
CASPubMed Google Scholar
- 13
Lessene, G. et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat. Chem. Biol.9, 390–397 (2013).
CASPubMed Google Scholar
- 14
Tao, Z.F. et al. Discovery of a potent and selective BCL-XL inhibitor with in vivo activity. ACS Med. Chem. Lett.5, 1088–1093 (2014).
CASPubMedPubMed Central Google Scholar
- 15
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res.68, 3421–3428 (2008).
CASPubMed Google Scholar
- 16
Bruncko, M. et al. Structure-guided design of a series of MCL-1 inhibitors with high affinity and selectivity. J. Med. Chem.58, 2180–2194 (2015).
CASPubMed Google Scholar
- 17
Cohen, N.A. et al. A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chem. Biol.19, 1175–1186 (2012).
CASPubMedPubMed Central Google Scholar
- 18
Kotschy, A. et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature538, 477–482 (2016).
PubMed Google Scholar
- 19
Leverson, J.D. et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis.6, e1590 (2015).
CASPubMedPubMed Central Google Scholar
- 20
Pelz, N.F. et al. Discovery of 2-indole-acylsulfonamide myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods. J. Med. Chem.59, 2054–2066 (2016).
CASPubMedPubMed Central Google Scholar
- 21
Stewart, M.L., Fire, E., Keating, A.E. & Walensky, L.D. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat. Chem. Biol.6, 595–601 (2010).
CASPubMedPubMed Central Google Scholar
- 22
Huhn, A.J., Guerra, R.M., Harvey, E.P., Bird, G.H. & Walensky, L.D. Selective covalent targeting of anti-apoptotic BFL-1 by cysteine-reactive stapled peptide inhibitors. Cell. Chem. Biol.23, 1123–1134 (2016).
CASPubMedPubMed Central Google Scholar
- 23
Gavathiotis, E., Reyna, D.E., Bellairs, J.A., Leshchiner, E.S. & Walensky, L.D. Direct and selective small-molecule activation of proapoptotic BAX. Nat. Chem. Biol.8, 639–645 (2012).
CASPubMedPubMed Central Google Scholar
- 24
Brahmbhatt, H., Uehling, D., Al-Awar, R., Leber, B. & Andrews, D. Small molecules reveal an alternative mechanism of Bax activation. Biochem. J.473, 1073–1083 (2016).
CASPubMedPubMed Central Google Scholar
- 25
Xin, M. et al. Small-molecule Bax agonists for cancer therapy. Nat. Commun.5, 4935 (2014).
CASPubMedPubMed Central Google Scholar
- 26
Zhao, G. et al. Activation of the proapoptotic Bcl-2 protein Bax by a small molecule induces tumor cell apoptosis. Mol. Cell. Biol.34, 1198–1207 (2014).
PubMedPubMed Central Google Scholar
- 27
Wang, K., Gross, A., Waksman, G. & Korsmeyer, S.J. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell. Biol.18, 6083–6089 (1998).
CASPubMedPubMed Central Google Scholar
- 28
Scott, D.E., Coyne, A.G., Hudson, S.A. & Abell, C. Fragment-based approaches in drug discovery and chemical biology. Biochemistry51, 4990–5003 (2012).
CASPubMed Google Scholar
- 29
Mayer, M. & Meyer, B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. Int. Ed. Engl.38, 1784–1788 (1999).
CASPubMed Google Scholar
- 30
Stockman, B.J. & Dalvit, C. NMR screening techniques in drug discovery and drug design. Prog. Nucl. Magn. Reson. Spectrosc.41, 187–231 (2002).
CAS Google Scholar
- 31
Tan, C. et al. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J. Biol. Chem.281, 14764–14775 (2006).
CASPubMedPubMed Central Google Scholar
- 32
Wei, M.C. et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev.14, 2060–2071 (2000).
CASPubMedPubMed Central Google Scholar
- 33
Pagliari, L.J. et al. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc. Natl. Acad. Sci. USA102, 17975–17980 (2005).
CASPubMed Google Scholar
- 34
Sarosiek, K.A. et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol. Cell51, 751–765 (2013).
What’s New in the PC-BaX Server Edition 3.0 serial key or number?
Screen Shot
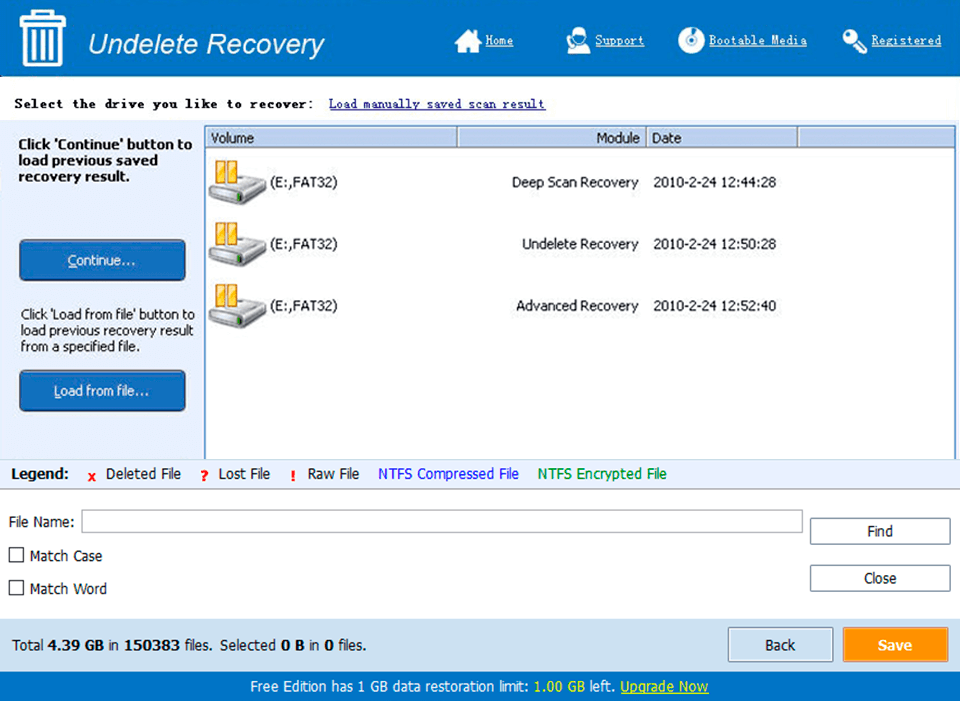
System Requirements for PC-BaX Server Edition 3.0 serial key or number
- First, download the PC-BaX Server Edition 3.0 serial key or number
-
You can download its setup from given links:


