
Internet Download Accelerator v2.0.1.532 serial key or number
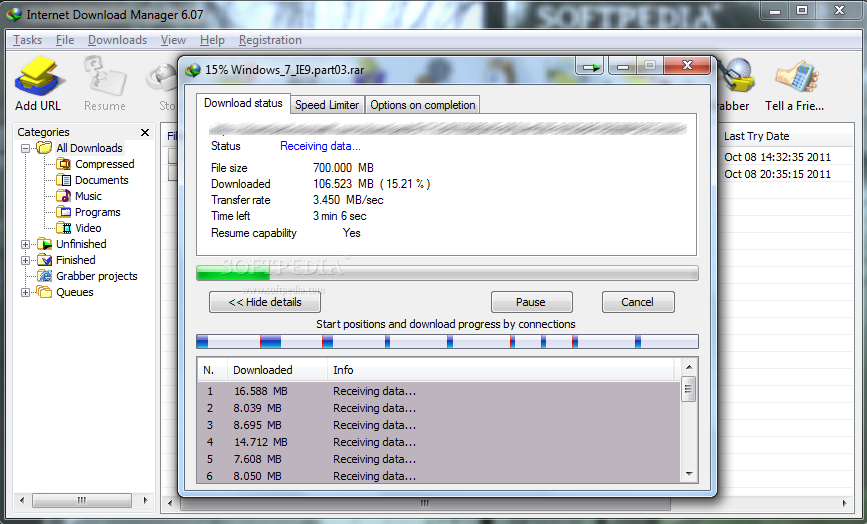
Internet Download Accelerator v2.0.1.532 serial key or number
NXS Form 63-2600, Users Manual, Technical Data - CONTROL ...
NX Series
Constant and Variable Torque Variable
Speed Drives for Induction Motors
63-2600—1
REFER TO THE START-UP QUICK GUIDE BELOW DURING INSTALLATION AND COMMISSIONING.
IF ANY PROBLEMS OCCUR, PLEASE CONTACT YOUR LOCAL DISTRIBUTOR.
Start-up Quick Guide
1. Check that the delivery corresponds to your order, see Chapter 3.
2. Before taking any commissioning actions read carefully the safety instructions in Chapter
1.
3. Before the mechanical installation, check the minimum clearances around the unit and
check the ambient conditions in Chapter 5.
4. Check the size of the motor cable, mains cable, mains fuses and check the cable
connections, read Chapters 6.1.1.1 to 6.1.1.5..
5. Follow the installation instructions, see Chapter 6.1.5.
6. Control connections are explained in Chapter 6.2.1.
7. If the Start-Up wizard is active, select the language of the keypad and the application
you want to use and confirm by pressing the Enter button. If the Start-Up wizard is not
active, follow the instructions 7a and 7b.
7a. Select the language of the keypad from the Menu M6, page 6.1. Instructions on using
the keypad are given in Chapter 7.
7b. Select the application you want to use from the Menu M6, page 6.2. Instructions on
using the keypad are given in Chapter 7.
8. All parameters have factory default values. In order to ensure proper operation, check
the rating plate data for the values below and the corresponding parameters of
parameter group G2.1.
• nominal voltage of the motor
• nominal frequency of the motor
• nominal speed of the motor
• nominal current of the motor
• motor cosϕ
All parameters are explained in the All in One Application Manual.
9. Follow the commissioning instructions, see Chapter 8.
10. NX_ Frequency Drive is now ready for use.
The Manufacturer is not responsible for the use of the frequency drives
outside the instructions provided.
CONTENTS
NX USER’S MANUAL
INDEX
1 SAFETY
2 EU DIRECTIVE
3 RECEIPT OF DELIVERY
4 TECHNICAL DATA
5 INSTALLATION
6 CABLING AND CONNECTIONS
7 CONTROL KEYPAD
8 COMMISSIONING
9 FAULT TRACING
THE NX FREQUENCY DRIVE USER'S MANUAL
AND THE APPLICATION MANUAL
The User's Manual will provide the necessary information about the installation, commissioning
and operation of NX Frequency Drives. It is recommended that these instructions are studied,
before powering up the frequency drive for the first time.
The Application Manual provides information about the different applications included in the
standard frequency drive. Should these applications not meet the requirements of the process,
contact Honeywell for information on special applications.
This manual is available in both paper and electronic editions. It is recommended that the
electronic version be used where possible as it contains several links and cross-references to
other locations in the manual which makes it easier for the reader to move around in the manual,
to check and find things faster.
NX User's Manual
Index
1. SAFETY....................................................................................................................................6
1.1 WARNINGS..............................................................................................................................................6
1.2 SAFETY INSTRUCTIONS............................................................................................................................6
1.3 GROUNDING AND GROUND FAULT PROTECTION.........................................................................................7
1.4 RUNNING THE MOTOR..............................................................................................................................7
2. DIRECTIVES.............................................................................................................................8
2.1 CE MARKING...........................................................................................................................................8
2.2 EMC DIRECTIVE......................................................................................................................................8
2.2.1 General...............................................................................................................................................8
2.2.2 Technical criteria ................................................................................................................................8
2.2.3 NX frequency drive EMC classification...............................................................................................8
2.2.4 Manufacturer's declaration of conformity............................................................................................9
2.3 UL-LABEL................................................................................................................................................9
3. RECEIPT OF SHIPMENT .......................................................................................................13
3.1 TYPE DESIGNATION CODE ......................................................................................................................13
3.2 STORAGE..............................................................................................................................................13
3.3 MAINTENANCE.......................................................................................................................................14
3.4 WARRANTY ...........................................................................................................................................14
4. TECHNICAL DATA ................................................................................................................15
4.1 INTRODUCTION......................................................................................................................................15
4.2 POWER RATINGS ...................................................................................................................................17
4.2.1 NX_5 – Mains voltage 380—500 V ..................................................................................................17
4.2.2 NX_6 – Mains voltage 525—690 V ..................................................................................................18
4.2.3 NX_2 – Mains voltage 208—240 V ..................................................................................................19
4.3 BRAKE RESISTOR RATINGS ....................................................................................................................20
4.4 TECHNICAL DATA...................................................................................................................................22
5. INSTALLATION......................................................................................................................24
5.1 MOUNTING ............................................................................................................................................24
5.2 COOLING...............................................................................................................................................34
5.2.1 FR4 to FR9 .......................................................................................................................................34
5.2.2 Standalone units (FR10 to FR12).....................................................................................................35
5.3 POWER LOSS ........................................................................................................................................36
5.3.1 Power loss as function of switching frequency .................................................................................36
6. CABLING AND CONNECTIONS............................................................................................40
6.1 POWER UNIT .........................................................................................................................................40
6.1.1 Power connections ...........................................................................................................................40
6.1.1.1 Mains and motor cables ................................................................................................................40
6.1.1.2 DC supply and brake resistor cables.............................................................................................41
6.1.1.3 Control cable .................................................................................................................................41
6.1.1.4 Cable and fuse sizes, NXS B and NXS A......................................................................................41
6.1.1.5 Cable and fuse sizes, NXS C ........................................................................................................42
6.1.1.6 Cable and fuse sizes, NX_A, FR10 to FR12 .................................................................................42
6.1.1.7 Cable and fuse sizes, NX_C, FR10 to FR12 .................................................................................43
6.1.2 Understanding the power unit topology ............................................................................................43
6.1.3 Changing EMC protection class from H to T ....................................................................................44
6.1.4 Mounting of cable accessories .........................................................................................................46
6.1.5 Installation instructions .....................................................................................................................48
6.1.5.1 Stripping lengths of motor and mains cables.................................................................................50
6.1.5.2 NX frequency drive frames and installation of cables....................................................................51
6.1.6 Cable installation and the UL standards...........................................................................................58
6.1.7 Cable and motor insulation checks...................................................................................................59
6.2 CONTROL UNIT ......................................................................................................................................60
6.2.1 NXS and NXP single phase input applications 380-500 VAC ..........................................................61
6.2.2 Control connections..........................................................................................................................62
6.2.2.1 Control cables................................................................................................................................63
6.2.2.2 Galvanic isolation barriers .............................................................................................................63
6.2.3 Control terminal signals ....................................................................................................................65
6.2.3.1 Digital input signal inversions ........................................................................................................66
6.2.3.2 Jumper selections on the OPT-A1 basic board .............................................................................67
7. CONTROL KEYPAD ..............................................................................................................69
7.1 INDICATIONS ON THE KEYPAD DISPLAY ...................................................................................................69
7.1.1 Drive status indications.....................................................................................................................69
7.1.2 Control place indications ..................................................................................................................70
7.1.3 Status LEDs (green – green – red)...................................................................................................70
7.1.4 Text lines ..........................................................................................................................................71
7.2 KEYPAD PUSH-BUTTONS........................................................................................................................72
7.2.1 Button descriptions ...........................................................................................................................72
7.3 NAVIGATION ON THE CONTROL KEYPAD ..................................................................................................73
7.3.1 Monitoring menu (M1) ......................................................................................................................75
7.3.2 Parameter menu (M2) ......................................................................................................................76
7.3.3 Keypad control menu (M3) ...............................................................................................................78
7.3.3.1 Selection of control place ..............................................................................................................78
7.3.3.2 Keypad reference ..........................................................................................................................79
7.3.3.3 Keypad direction............................................................................................................................79
7.3.3.4 Stop button activated.....................................................................................................................79
7.3.4 Active faults menu (M4)....................................................................................................................80
7.3.4.1 Fault types .....................................................................................................................................81
7.3.4.2 Fault codes....................................................................................................................................82
7.3.4.3 Fault time data record....................................................................................................................84
7.3.5 Fault history menu (M5)....................................................................................................................85
7.3.6 System menu (M6) ...........................................................................................................................86
7.3.6.1 Language selection .......................................................................................................................89
7.3.6.2 Application selection......................................................................................................................89
7.3.6.3 Parameter copy .............................................................................................................................90
7.3.6.4 Parameter comparison ..................................................................................................................92
7.3.6.5 Security..........................................................................................................................................93
7.3.6.6 Keypad settings .............................................................................................................................95
7.3.6.7 Hardware settings..........................................................................................................................96
7.3.6.8 System info....................................................................................................................................99
7.3.7 Expander board menu (M7)............................................................................................................103
7.4 FURTHER KEYPAD FUNCTIONS .............................................................................................................104
8. COMMISSIONING ................................................................................................................105
8.1 SAFETY...............................................................................................................................................105
8.2 COMMISSIONING OF THE FREQUENCY DRIVE.........................................................................................105
9. FAULT TRACING .................................................................................................................108
1
6(110) Safety
1. SAFETY
ONLY A COMPETENT ELECTRICIAN SHOULD CARRY OUT
THE ELECTRICAL INSTALLATION
1.1 Warnings
WARNING
1
The NX frequency drive is meant for fixed installations only.
2
Do not perform any measurements when the frequency drive is
connected to the mains. The motor terminals U, V, W and the DClink/brake
resistor terminals –/+ are live when the NX is connected to
mains, even if the motor is not running.
3
Do not perform any voltage withstand tests on any part of the NX.
4
The frequency drive has a large capacitive leakage current.
5
If the frequency drive is used as a part of a machine, the machine
manufacturer is responsible for providing the machine with a main switch
(EN 60204-1).
6
Only spare parts delivered by Honeywell can be used.
7
The motor starts at power-up if the start command is 'ON'. Furthermore,
the I/O functionalities (including start inputs) may change if parameters,
applications or software are changed. Disconnect, therefore, the motor if
an unexpected start can cause danger.
8
Prior to measurements on the motor or the motor cable, disconnect the
motor cable from the frequency drive.
9
Do not touch the IC-circuits on the circuit boards. Static voltage discharge
may damage the components.
1.2 Safety instructions
1
The components of the power unit of the frequency drive are live when
the NX is connected to mains potential. ontact with this voltage is
extremely dangerous and may cause death or severe injury. The
control unit is isolated from the potential.
2
The motor terminals U, V, W and the DC-link/brake resistor terminals –/+
are live when the NX is connected to mains, even if the motor is not
running.
3
After disconnecting the frequency drive from the mains, wait until the fan
stops and the indicators on the keypad extinguish. (if no keypad is
attached see the indicators on the cover). Wait 5 more minutes before
doing any work on the NX connections. Do not even open the cover
before this time has expired.
4
The control I/O-terminals are isolated from the mains potential. However,
the relay outputs and other I/O-terminals may have a dangerous control
voltage present even when the NX is disconnected from mains.
5
Before connecting the frequency drive to mains, ensure that the
frequency drive front and cable covers are closed.
Safety 7(110)
1
1.3 Grounding and ground fault protection
The NX frequency drive must always be grounded via a conductor connected to the grounding
terminal .
The ground fault protection inside the frequency drive protects only the drive itself against ground
faults in the motor or the motor cable.
If fault current protective switches (e.g. RCD or Ground Leakage devices) are to be used in
conjunction with the frequency drive, they must be tested with ground fault currents that are
possible to arise in fault situations.
1.4 Running the motor
Warning symbols
For your own safety please pay special attention to the instructions marked with the following
symbols:
= Dangerous voltage
WARNING
= General warning
HOT SURFACE
= Hot surface – Risk of burn
MOTOR RUN CHECK LIST
1
Before starting the motor, check that the motor is mounted properly
and ensure that the machine connected to the motor allows the
motor to be started.
2
Set the maximum motor speed (frequency) according to the motor
and the machine connected to it.
3
Before reversing the motor shaft rotation direction make sure that
WARNING this can be done safely.
4
Ensure that no power correction capacitors are connected to the
motor cable.
5
Ensure that the motor terminals are not connected to mains
potential.
8(110) DIRECTIVES
2. DIRECTIVES
2.1 CE marking
The CE marking on the product guarantees the free movement of the product within the EEA
(European Economic Area). It also guarantees that the product meets the various requirements
defined by the directive.
The NX frequency drives carry the CE label as a proof of compliance with the Low Voltage
Directive (LVD) and the Electro Magnetic Compatibility (EMC). The company SGS FIMKO has
acted as the Competent Body.
2.2 EMC directive
2.2.1 General
The EMC Directive provides that the electrical apparatus must not excessively disturb the
environment it is used in, and also, it shall have an adequate level of immunity toward other
disturbances from the same environment.
The compliance of the NX frequency drives with the EMC directive is verified with Technical
Construction Files (TCF) checked and approved by SGS FIMKO, which is a Competent Body. The
Technical Construction Files are used to authenticate the comformity of the NX frequency drives
with the Directive due to the large product family & variety of installations possibilities.
2.2.2 Technical criteria
The NX frequency drives are marketed throughout the world, a fact which makes the EMC
requirements of customers different. As far as the immunity is concerned, all NX frequency drives
are designed to fulfil even the strictest requirements, while as regards the emission level, the
customer may want to upgrade the NX's already high ability to filter electro-magnetic
disturbances.
2.2.3 NX frequency drive EMC classification
The NX frequency drives are divided into three classes, according to the level of electromagnetic
disturbances emitted. There is no difference in the functions or the control electronics between
these classes but their EMC properties vary as follows:
Class H:
NX_5 frequency drives (FR4 to FR9) and NX_2 frequency drives (FR4 to FR6) have been
designed to fulfil the requirements of the product standard IEC 61800-3+A11 for the 1st
environment restricted distribution and the 2nd environment.
The emission levels correspond to the requirements of IEC 61000-6-4.
Class L (NX_5, FR10 only):
Provides filtering for the 2 nd environment, restricted distribution according to IEC 61800-3+A11.
2
DIRECTIVES 9(110)
Class T:
The T-class drives have a small ground current and can be used with IT supplies only. If they are
used with other supplies no EMC requirements are complied with.
Class N:
The drives of this class do not provide EMC emission protection. This kind of drives are mounted
in enclosures.
All NX frequency drives fulfil all EMC immunity requirements (standards IEC 61000-6-1,
61000-6-2 and IEC 61800-3+A11).
Warning: This is a product of the restricted sales distribution class according to IEC 61800-
3. In a domestic environment this product may cause radio interference in which case the
user may be required to take adequate measures.
Note: For changing the EMC protection class of your NX frequency drive from class H to class T,
please refer to the instructions given in Chapter 6.3.1.
2.2.4 Manufacturer's declaration of conformity
The following pages present the photocopies of the Manufacturer's Declarations of Conformity
assuring the compliance of the NX frequency drives with the EMC-directives.
2.3 UL-label
The NX frequency drives are UL-listed according to the standards, based on the needed voltage
and power range. For more information contact you local Honeywell distributor. More information
of cable selection and installation can be found from chapter 5 and 6.
2
10(110) DIRECTIVES
We
Manufacturer's name:
EU DECLARATION OF CONFORMITY
Vacon Oyj
Manufacturer's address: P.O.Box 25
Runsorintie 7
FIN-65381 Vaasa
Finland
hereby declare that the product
Product name:
Model designation:
NXS/P Frequency converter
NXS/P 0003 5…. to 0520 5….
has been designed and manufactured in accordance with the following
standards:
Safety: EN50178 (1997), EN60204-1 (1996)
EN 60950 (3rd edition 2000, as relevant)
EMC: EN61800-3 (1996)+A11(2000), EN 61000-6-2
(1999), EN 61000-6-4 (2001)
and conforms to the relevant safety provisions of the Low Voltage Directive
(73/23/EEC) as amended by the Directive (93/68/EEC) and EMC Directive
89/336/EEC.
It is ensured through internal measures and quality control that the product
conforms at all times to the requirements of the current Directive and the
relevant standards.
In Vaasa, 5th of May, 2003
Vesa Laisi
President
The year the CE marking was affixed: 2002
2
Receipt of shipment 11(110)
3
We
Manufacturer's name:
EU DECLARATION OF CONFORMITY
Vacon Oyj
Manufacturer's address: P.O.Box 25
Runsorintie 7
FIN-65381 Vaasa
Finland
hereby declare that the product
Product name:
NXS/P Frequency converter
Model designation:
NXS/P 0004 6…. to 0416 6….
has been designed and manufactured in accordance with the following
standards:
Safety: EN50178 (1997), EN60204-1 (1996)
EN 60950 (3rd edition 2000, as relevant)
EMC: EN61800-3 (1996)+A11(2000), EN 61000-6-2
(1999), EN 61000-6-4 (2001)
and conforms to the relevant safety provisions of the Low Voltage Directive
(73/23/EEC) as amended by the Directive (93/68/EEC) and EMC Directive
89/336/EEC.
It is ensured through internal measures and quality control that the product
conforms at all times to the requirements of the current Directive and the
relevant standards.
In Vaasa, 17th of November, 2003
Vesa Laisi
President
The year the CE marking was affixed: 2003
3
12(110) Receipt of shipment
We
Manufacturer's name:
EU DECLARATION OF CONFORMITY
Vacon Oyj
Manufacturer's address: P.O.Box 25
Runsorintie 7
FIN-65381 Vaasa
Finland
hereby declare that the product
Product name:
NXS/P Frequency converter
Model designation:
NXS/P 0003 2…. to 0114 2….
has been designed and manufactured in accordance with the following
standards:
Safety: EN50178 (1997), EN60204-1 (1996)
EN 60950 (3rd edition 2000, as relevant)
EMC: EN61800-3 (1996)+A11(2000), EN 61000-6-2
(1999), EN 61000-6-4 (2001)
and conforms to the relevant safety provisions of the Low Voltage Directive
(73/23/EEC) as amended by the Directive (93/68/EEC) and EMC Directive
89/336/EEC.
It is ensured through internal measures and quality control that the product
conforms at all times to the requirements of the current Directive and the
relevant standards.
In Vaasa, 10th of November, 2003
Vesa Laisi
President
The year the CE marking was affixed: 2003
Note: Ask factory for other possible installation combinations.
Receipt of shipment 13(110)
3
3. RECEIPT OF SHIPMENT
The NX frequency drives have undergone rigorous tests and quality checks at the factory before
delivery. However, after unpacking the product, check that no signs of transport damages are to
be found on the product and that the delivery is complete (compare the type designation of the
product to the code below, Figure 3-1.
Should the drive have been damaged during the shipping, contact the carrier and or distributor.
If the delivery does not correspond to your order, contact the supplier immediately.
In the small plastic bag included in the delivery you will find a silver Drive modified sticker. The
purpose of the sticker is to notify the service personnel about the modifications made in the
frequency drive. Attach the sticker on the side of the frequency drive to avoid losing it. Should the
frequency drive be later modified (option board added, IP or EMC protection level changed), mark
the change in the sticker.
3.1 Type designation code
N X S 0 0 5 0 B 1 2
E n c l osure 00 Open chassis
10 Nema 1
12 Nema 12
V o l t a ge range B 208-240 Vac 3 p h a s e
A 380-500 Vac 3 p h a s e
C 525-690 Vac 3 p h a s e
M o t or Power (HP) 0005 1/2 HP
L o w overloadability: 0050 5 HP
1 0 % overload at 0400 40 HP e t c
1 0 4 deg F
Figure 3-1. NX type designation code
P r o duct Series
NXS
NXL
3.2 Storage
If the frequency drive is to be kept in store ensure that the ambient conditions are acceptable:
Storing temperature -40…+158°F (-40…70° C)
Relative humidity
3
14(110) Receipt of shipment
3.3 Maintenance
In normal conditions, the NX frequency drives are maintenance-free. However, it is recommended
the heatsink be cleared periodically with compressed air. The cooling fan can easily be changed if
necessary.
It may also be necessary to check the tightening torques of terminals at regular intervals.
3.4 Warranty
Only manufacturing defects are covered by the warranty. The manufacturer assumes no
responsibility for damages caused during or resulting from transport, receipt of the delivery,
installation, commissioning or use.
The manufacturer shall in no event and under no circumstances be held responsible for damages
and failures resulting from misuse, incorrect installation, unacceptable ambient temperature, dust,
corrosive substances or operation outside the rated specifications.
Neither can the manufacturer be held responsible for consequential damages.
The Manufacturer's period of warranty is 18 months from the delivery or 12 months from the
commissioning whichever expires first.
The local distributor may grant a warranty time different from the above. This warranty period shall
be specified in the distributor's sales and warranty terms. The manufacturer assumes no
responsibility for warranties offered by others. With all warranty issues, please contact the
distributor first.
Technical data 15(110)
4. TECHNICAL DATA
4.1 Introduction
Figure 4-1 presents the block diagram of the NX frequency drive. The frequency drive consists of
two units, the Power Unit and the Control Unit.
The three-phase AC-choke (1) at the mains end together with the DC-link capacitor (2) form an
LC-filter, which, again, together with the diode bridge produce the DC-voltage supply to the IGBT
Inverter Bridge (3) block. The AC-choke also functions as a filter against High Frequency
disturbances from the mains as well as against those caused by the frequency drive to the mains.
It, in addition, enhances the waveform of the input current to the frequency drive. The entire power
drawn by the frequency drive from the mains is active power.
The IGBT Inverter Bridge produces a symmetrical, 3-phase PWM-modulated AC-voltage to the
motor.
The Motor and Application Control Block is based on microprocessor software. The
microprocessor controls the motor basing on the information it receives through measurements,
parameter settings, control I/O and control keypad. The motor and application control block
controls the motor control ASIC which, in turn, calculates the IGBT positions. Gate drivers amplify
these signals for driving the IGBT inverter bridge.
Power
module
Brake resistor*
Mains
L1
L2
L3
1)
Integrated input module
Rectifier
3~
=
Charg.res.
Brake
Chopper*
2)
3)
IGBT
Inverter Current
Sensors
=
3~
Motor
U Output
V EMCfilter
W
Fan
Power
Supply
Measurements
Gate
Drivers
Voltage
Sensors
NXP
Control
Keypad
Control
module
Motor and
Application
Control
Motor
Control
ASIC
Control
I/O
Control
I/O
Control
I/O
Control
I/O
Control
I/O
*The brake resistor can be installed internally in classes FR4 to FR6 (NX_2 and NX_5). In all other
frames of voltage classes NX_2 and NX_5, as well as in all frames of all other voltage classes, the
brake resistor is available as option and installed externally.
Brake chopper belongs to the standard equipment in classes FR4 to FR6, while in greater classes
(FR7 to FR9) it is optional.
Figure 4-1. NX block diagram
Automation and Control Solutions
Honeywell
Honeywell Limited-Honeywell Limitée
1985 Douglas Drive North 35 Dynamic Drive
Golden Valley, MIN 55422 Scarborough, Ontario 63-2600-1
MIV 4Z9
www.honeywell.com
4
16(110) Technical data
The control keypad provides a link between the user and the frequency drive. The control keypad
is used for parameter setting, reading status data and giving control commands. It is detachable
and can be operated externally and connected via a cable to the frequency drive. Also a PC can
be used instead of the control keypad, to control the frequency drive, if connected through a
similar cable.
Control I/O boards which are either isolated (OPT-A8) or not isolated (OPT-A1) from the ground
are available.
The default application (Basic Application) is preferred when speed control will be dictated by a
separate automation system. If a more versatile interface or parameters are required, a more
suitable application can be chosen from the Application Package. See the Application Manual for
more information on the different applications.
A brake resistor is available as internal option for frames FR4 to FR6 of voltage classes NX_2 and
NX_5. In all other frames of voltage classes NX_2 and NX_5, as well as in all frames of all other
voltage classes, the brake resistor is available as option and installed externally.
Optional I/O expander boards that increase the number of inputs and outputs to be used are also
available. For details please contact your nearest Honeywell office or your local distributor (see
back cover).
The input and output EMC filters have no influence on the basic functions of the frequency drives
and significantly enhance the protection of the drive from external interference as well as
protecting other sensitive equipment from harmonics generated by the frequency drive. They are
also necessary for the fulfillment of the EMC directives.
4
Automation and Control Solutions
Honeywell
Honeywell Limited-Honeywell Limitée
1985 Douglas Drive North 35 Dynamic Drive
Golden Valley, MIN 55422 Scarborough, Ontario 63-2600-1
MIV 4Z9
www.honeywell.com
Technical data 17(110)
4.2 Power ratings
4.2.1 NX_5 – Mains voltage 380—500 V
Low overload = 150% starting torque, 2 sec/20 sec, 110% overloadability, 1 min/10 min
Following continuous operation at rated output current, 110% rated output current (IL)
for 1 min, followed by a period of load current less than rated current, and of such
duration that the r.m.s output current, over the duty cycle, does not exceed rated output
current (IL)
High overload = 200% starting torque, 2 sec/20 sec, 150% overloadability, 1 min/10 min
Following continuous operation at rated output current, 150 % rated output current (IH)
for 1 min, followed by a period of load current less than rated current, and of such
duration that the r.m.s output current, over the duty cycle, does not exceed rated output
current (IH)
All sizes up to and including FR9 are wall mounted available with NEMA1 enclosure and
NEMA12 as option. All sizes above and including FR10 are standalone NEMA 1 NXP units.
For single phase input connections, ratings and wiring instructions can be found in 6.2.1
Mains voltage 380-500 V, NEMA 1/12, EMC-level H
Frequency Motor shaft power (500V) and current
drive type Low overload High overload
P [Hp]
(500V)
I(L)
P [Hp]
(500V)
I(H)
I(max)
Size / prot. FR/IP
Dimensions
WxHxD
(in)
Weight
(lb)
NX_ 0015 A 1.5 3.3 1 2.2 4.4 FR4/NEMA 1/12 5.0x11.5x7.5 11.02
NX_ 0020 A 2 4.3 1.5 3.3 6.2 FR4/NEMA 1/12 5.0x11.5x7.5 11.02
NX_ 0030 A 3 5.6 2 4.3 8.6 FR4/NEMA 1/12 5.0x11.5x7.5 11.02
NX_ 0040 A 4 7.6 3 5.6 10.8 FR4/NEMA 1/12 5.0x11.5x7.5 11.02
NX_ 0050 A 5 9 4 7.6 14 FR4/NEMA 1/12 5.0x11.5x7.5 11.02
NX_ 0075 A 7.5 12 5 9 18 FR4/NEMA 1/12 5.0x11.5x7.5 11.02
NX_ 0100 A 10 16 7.5 12 24 FR5/NEMA 1/12 5.7x15.4x8.4 17.86
NX_ 0150 A 15 23 10 16 32 FR5/NEMA 1/12 5.7x15.4x8.4 17.86
NX_ 0200 A 20 31 15 23 46 FR5/NEMA 1/12 5.7x15.4x8.4 17.86
NX_ 0250 A 25 38 20 31 62 FR6/NEMA 1/12 7.7x20.4x9.3 40.8
NX_ 0300 A 30 46 25 38 76 FR6/NEMA 1/12 7.7x20.4x9.3 40.8
NX_ 0400 A 40 61 30 46 92 FR6/NEMA 1/12 7.7x20.4x9.3 40.8
NX_ 0500 A 50 72 40 61 122 FR7/NEMA 1/12 9.3x23.3x10.1 77.2
NX_ 0600 A 60 87 50 72 144 FR7/NEMA 1/12 9.3x23.3x10.1 77.2
NX_ 0750 A 75 105 60 87 174 FR7/NEMA 1/12 9.3x23.3x10.1 77.2
NX_ 1000 A 100 140 75 105 210 FR8/NEMA 1/12 11.2x28.4x11.3 127.9
NX_ 1250 A 125 170 100 140 280 FR8/NEMA 1/12 11.2x28.4x11.3 127.9
NX_ 1500 A 150 205 125 170 336 FR8/NEMA 1/12 11.2x28.4x11.3 127.9
NX_ 2000 A 200 261 150 205 349 FR9/NEMA 1/12 18.9x45.3x14.3 321.9
NX_ 2500 A 250 300 200 245 444 FR9/NEMA 1/12 18.9x45.3x14.3 321.9
NXP 3000 A 300 385 250 300 540 FR10/NEMA1 23.6x89.6x23.6 661.1
NXP 3500 A 350 460 300 385 693 FR10/NEMA1 23.6x89.6x23.6 661.1
NXP 4500 A 450 520 350 460 828 FR10/NEMA1 23.6x89.6x23.6 661.1
NXP 5000 A 500 590 450 520 936 FR11/NEMA1 31.6x79.4x23.6 815.7
NXP 5500 A 550 650 500 590 1062 FR11/NEMA1 31.6x79.4x23.6 815.7
NXP 6000 A 600 730 550 650 1170 FR11/NEMA1 31.6x79.4x23.6 815.7
NXP 6500 A 650 820 600 730 1314 FR12/NEMA1 47.6x 79.4x 23.6 1322.8
NXP 7000 A 700 920 650 820 1476 FR12/NEMA1 47.6x 79.4x 23.6 1322.8
NXP 8000 A 800 1030 700 920 1654 FR12/NEMA1 47.6x 79.4x 23.6 1322.8
Table 4-1. Power ratings and dimensions of the NX, supply voltage 380—500V.
Note: The rated currents in given ambient temperatures are achieved only when the switching frequency is
equal to or less than the factory default.
Note: The rated currents for FR10 to FR12 are all valid at an ambient temperature of 104 °F.
Automation and Control Solutions
Honeywell
Honeywell Limited-Honeywell Limitée
1985 Douglas Drive North 35 Dynamic Drive
Golden Valley, MIN 55422 Scarborough, Ontario 63-2600-1
MIV 4Z9
www.honeywell.com
4
18(110) Technical data
4.2.2 NX_6 – Mains voltage 525—690 V
High overload = Max current IS, 2 sec/20 sec, 150% overloadability, 1 min/10 min
Following continuous operation at rated output current, 150 % rated output current (IH)
for 1 min, followed by a period of load current less than rated current, and of such
duration that the r.m.s output current, over the duty cycle, does not exceed rated output
current (IH)
Low overload = Max current IS, 2 sec/20 sec, 110% overloadability, 1 min/10 min
Following continuous operation at rated output current, 110% rated output current (IL)
for 1 min, followed by a period of load current less than rated current, and of such
duration that the r.m.s output current, over the duty cycle, does not exceed rated output
ORIGINAL RESEARCH Impact of pretreatment PET ... - Surgery News
ORIGINALRESEARCH
ImpactofpretreatmentPET on disease control
and treatment decisions in locoregionally
advanced esophageal cancer patients treated
with chemoradiotherapy
John M. Watkins et al p. 8
Variation by age in neutropenic
complications among patients with
cancer receiving chemotherapy
Henry J. Henk et al p. 16
COMMENTARY
Regorafenib monotherapy for previously
treated metastatic colorectal cancer
Stuart M. Lichtman, MD p. 3
COMMUNITY TRANSLATIONS
Regorafenib in previously treated
metastatic colorectal cancer
Edited by Jame Abraham, MD p. 5
FEATURES
Technology: LinkedIn Facebook
HIPPA friendly and secure Doximity
Neil Osterweil p. 33
Volume 10 ● Number 1 ● January 2013
There is an association between
pretreatmentPET and disease
control endpoints, including
locoregional control in esophageal
tumors treated with
chemoradiotherapy.
John M. Watkins p. 8
Oncologists should evaluate patient
comorbidities and chemotherapy
characteristics as well as age when
assessing the risks for neutropeniarelated
hospitalization.
Henry J. Henk p. 16
One of the caveats for regorafenib for
previously treated metastatic
colorectal is that none of the patients
in the trial were in their 70s, which
is the age range of most patients
with this disease.
Stuart M. Lichtman p. 3
Complete table of contents, page A7
COMMUNITY ONCOLOGY January 2013
Volume 10, Number 1 (pp 1–34)
For chronic lymphocytic leukemia (CLL)
refractory to fludarabine and alemtuzumab
EXPAND YOUR OPTIONS
A study population in need of additional treatment options 1,2
5 median
prior therapies
59%
93%
100%
The following serious adverse events (AEs) are discussed in greater detail below:
Infusion reactions, cytopenias, progressive multifocal leukoencephalopathy, hepatitis B
infection and reactivation, and intestinal obstruction.
To learn more, please visit www.ARZERRA.com.
Indication
ARZERRA (ofatumumab) is indicated for the treatment
of patients with chronic lymphocytic leukemia (CLL)
refractory to fludarabine and alemtuzumab.
The effectiveness of ARZERRA is based on the
demonstration of durable objective responses. No
data demonstrate an improvement in disease-related
symptoms or increased survival with ARZERRA.
Important Safety Information
Infusion Reactions
ARZERRA can cause serious infusion reactions manifesting
as bronchospasm, dyspnea, laryngeal edema, pulmonary
edema, flushing, hypertension, hypotension, syncope,
cardiac ischemia/infarction, back pain, abdominal pain,
pyrexia, rash, urticaria, and angioedema. Infusion reactions
occur more frequently with the first 2 infusions. Premedicate
with acetaminophen, an antihistamine, and a corticosteroid.
Interrupt infusion for infusion reactions of any severity.
Institute medical management for severe infusion reactions
including angina, or other signs and symptoms of myocardial
ischemia. In a study of patients with moderate to severe
chronic obstructive pulmonary disease, an indication for
which ARZERRA is not approved, 2 of 5 patients developed
Grade 3 bronchospasm during infusion. Infusion reactions
occurred in 44% of patients on the day of the first infusion
(300 mg), 29% on the day of the second infusion (2,000 mg),
and less frequently during subsequent infusions.
of patients received prior rituximab
of patients received prior alkylating agents
of patients received prior fludarabine and alemtuzumab
Cytopenias
Prolonged (≥1 week) severe neutropenia and
thrombocytopenia can occur with ARZERRA. Monitor
complete blood counts (CBC) and platelet counts at regular
intervals during therapy, and increase the frequency of
monitoring in patients who develop Grade 3 or 4 cytopenias.
Of 108 patients with normal neutrophil counts at baseline,
45 (42%) developed ≥Grade 3 neutropenia. Nineteen (18%)
developed Grade 4 neutropenia. Some patients experienced
new onset Grade 4 neutropenia >2 weeks in duration.
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML),
including fatal PML, can occur with ARZERRA. Consider
PML in any patient with new onset of or changes in
pre-existing neurological signs or symptoms. Discontinue
ARZERRA if PML is suspected and initiate evaluation for
PML including consultation with a neurologist, brain MRI,
and lumbar puncture.
Hepatitis B Infection and Reactivation
Fulminant and fatal hepatitis B virus (HBV) infection and
reactivation can occur in patients following treatment with
ARZERRA. Screen patients at high risk of HBV infection
before initiation of ARZERRA. Closely monitor carriers of
hepatitis B for clinical and laboratory signs of active HBV
infection during treatment with ARZERRA and for 6 to 12
months following the last infusion of ARZERRA. Discontinue
ARZERRA in patients who develop viral hepatitis or
When treated with ARZERRA monotherapy, 42% of patients with
CLL refractory to fludarabine and alemtuzumab achieved a partial response 1
Overall response rate with ARZERRA
60
50
40
30
20
10
42%
FLUDARABINE AND
ALEMTUZUMAB REFRACTORY
(n=59)
reactivation of viral hepatitis, and institute appropriate
treatment. Insuffi cient data exist regarding the safety of
administration of ARZERRA in patients with active hepatitis.
Intestinal Obstruction
Obstruction of the small intestine can occur in patients
receiving ARZERRA. Perform a diagnostic evaluation if
obstruction is suspected.
Immunizations
The safety of immunization with live viral vaccines during or
following administration of ARZERRA has not been studied.
Do not administer live viral vaccines to patients who have
recently received ARZERRA. The ability to generate an
immune response to any vaccine following administration
of ARZERRA has not been studied.
Most Common Adverse Reactions
In the pivotal study (total population, n=154) the most
common adverse reactions (≥10%, all grades) were
neutropenia, followed by pneumonia (23%), pyrexia (20%),
cough (19%), diarrhea (18%), anemia (16%), fatigue (15%),
dyspnea (14%), rash (14%), nausea (11%), bronchitis (11%),
and upper respiratory tract infections (11%).
Most Common Serious Adverse Reactions
In the pivotal study (total population, n=154), where
ARZERRA was administered at 2,000 mg beginning with the
second dose for 11 doses, the most common serious adverse
Patients had received a median of 5 prior therapies
The investigator-determined overall response rate
in patients with CLL refractory to fl udarabine and
alemtuzumab was 42% (99% CI: 26, 60)
There were no complete responses
The eff ectiveness of ARZERRA is based on the
demonstration of durable objective responses
No data demonstrate an improvement in disease-related
symptoms or increased survival with ARZERRA
6.5 months—median duration of response
(95% CI: 5.8, 8.3)
reactions were infections (including pneumonia and sepsis),
neutropenia, and pyrexia.
A total of 108 patients (70%) experienced bacterial, viral, or
fungal infections. A total of 45 patients (29%) experienced
≥Grade 3 infections, of which 19 (12%) were fatal. The
proportion of fatal infections in the fludarabine- and
alemtuzumab-refractory group was 17%.
Please see Brief Summary of Prescribing Information on
adjacent pages.
How Supplied: Available as 2 diff erent single-use glass vials
for dilution and intravenous administration. Each vial contains
either 100 mg ofatumumab in 5 mL of solution or 1,000 mg
ofatumumab in 50 mL of solution.
References: 1. ARZERRA (ofatumumab) [package insert]. Research Triangle Park,
NC: GlaxoSmithKline; 2011. 2. Tam CS, O’Brien S, Lerner S, et al. Leuk Lymph.
2007;48(10):1931-1939.
BRIEF SUMMARY
ARZERRA ® (ofatumumab) Injection, for intravenous infusion
The following is a brief summary only; see full prescribing information for
complete product information.
1 INDICATIONS AND USAGE
ARZERRA ® (ofatumumab) is indicated for the treatment of patients
with chronic lymphocytic leukemia (CLL) refractory to fludarabine and
alemtuzumab. The effectiveness of ARZERRA is based on the demonstration
of durable objective responses [see Clinical Studies (14) of full prescribing
information]. No data demonstrate an improvement in disease-related
symptoms or increased survival with ARZERRA.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Infusion Reactions ARZERRA can cause serious infusion reactions
manifesting as bronchospasm, dyspnea, laryngeal edema, pulmonary edema,
flushing, hypertension, hypotension, syncope, cardiac ischemia/infarction,
back pain, abdominal pain, pyrexia, rash, urticaria, and angioedema. Infusion
reactions occur more frequently with the first 2 infusions [see Adverse
Reactions (6.1)]. Premedicate with acetaminophen, an antihistamine, and a
corticosteroid [see Dosage and Administration (2.1, 2.4) of full prescribing
information]. Interrupt infusion for infusion reactions of any severity. Institute
medical management for severe infusion reactions including angina or other
signs and symptoms of myocardial ischemia [see Dosage and Administration
(2.3) of full prescribing information]. In a study of patients with moderate
to severe chronic obstructive pulmonary disease, an indication for which
ARZERRA is not approved, 2 of 5 patients developed Grade 3 bronchospasm
during infusion. 5.2 Cytopenias Prolonged (≥1 week) severe neutropenia and
thrombocytopenia can occur with ARZERRA. Monitor complete blood counts
(CBC) and platelet counts at regular intervals during therapy, and increase
the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias.
5.3 Progressive Multifocal Leukoencephalopathy Progressive multifocal
leukoencephalopathy (PML), including fatal PML, can occur with ARZERRA.
Consider PML in any patient with new onset of or changes in pre-existing
neurological signs or symptoms. Discontinue ARZERRA if PML is suspected,
and initiate evaluation for PML including consultation with a neurologist,
brain MRI, and lumbar puncture. 5.4 Hepatitis B Infection and Reactivation
Fulminant and fatal hepatitis B virus (HBV) infection and reactivation can
occur in patients following treatment with ARZERRA. Screen patients at high
risk of HBV infection before initiation of ARZERRA. Closely monitor carriers
of hepatitis B for clinical and laboratory signs of active HBV infection during
treatment with ARZERRA and for 6 to 12 months following the last infusion
of ARZERRA. Discontinue ARZERRA in patients who develop viral hepatitis or
reactivation of viral hepatitis, and institute appropriate treatment. Insufficient
data exist regarding the safety of administration of ARZERRA in patients with
active hepatitis. 5.5 Intestinal Obstruction Obstruction of the small intestine
can occur in patients receiving ARZERRA. Perform a diagnostic evaluation
if obstruction is suspected. 5.6 Immunizations The safety of immunization
with live viral vaccines during or following administration of ARZERRA has
not been studied. Do not administer live viral vaccines to patients who have
recently received ARZERRA. The ability to generate an immune response to
any vaccine following administration of ARZERRA has not been studied.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in
other sections of the labeling:
• Infusion Reactions [see Warnings and Precautions (5.1)]
• Cytopenias [see Warnings and Precautions (5.2)]
• Progressive Multifocal Leukoencephalopathy [see Warnings and
Precautions (5.3)]
• Hepatitis B Reactivation [see Warnings and Precautions (5.4)]
• Intestinal Obstruction [see Warnings and Precautions (5.5)]
The most common adverse reactions (≥10%) in Study 1 were neutropenia,
pneumonia, pyrexia, cough, diarrhea, anemia, fatigue, dyspnea, rash,
nausea, bronchitis, and upper respiratory tract infections. The most common
serious adverse reactions in Study 1 were infections (including pneumonia
and sepsis), neutropenia, and pyrexia. Infections were the most common
adverse reactions leading to drug discontinuation in Study 1. 6.1 Clinical
Trials Experience Because clinical trials are conducted under widely varying
conditions, adverse reaction rates observed in the clinical trials of a drug
cannot be directly compared to rates in the clinical trials of another drug and
may not reflect the rates observed in practice. The safety of monotherapy
with ARZERRA was evaluated in 181 patients with relapsed or refractory
CLL in 2 open-label, non-randomized, single-arm studies. In these studies,
ARZERRA was administered at 2,000 mg beginning with the second dose
for 11 doses (Study 1 [n = 154]) or 3 doses (Study 2 [n = 27]). The data
described in Table 1 and other sections below are derived from 154 patients
in Study 1. All patients received 2,000 mg weekly from the second dose
onward. Ninety percent of patients received at least 8 infusions of ARZERRA
and 55% received all 12 infusions. The median age was 63 years (range: 41
to 86 years), 72% were male, and 97% were White.
Table 1. Incidence of All Adverse Reactions Occurring in ≥5% of Patients
in Study 1 and in the Fludarabine- and Alemtuzumab-Refractory Subset
of Study 1 (MedDRA 9.0)
Total Population
(n = 154)
All
Grades
%
Grade
≥3
%
Fludarabine- and
Alemtuzumab-
Refractory
(n = 59)
All
Grades
%
Grade
≥3
%
Body System/
Adverse Event
Infections and infestations
Pneumoniaa 23 14 25 15
Upper respiratory tract
infection
11 0 3 0
Bronchitis 11
7 DRUG INTERACTIONS
No formal drug-drug interaction studies have been conducted with ARZERRA.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy Pregnancy Category C: There are no adequate or well-controlled
studies ofofatumumab in pregnant women. A reproductive study in pregnant
cynomolgus monkeys that received ofatumumab at doses up to 3.5 times the
recommended human dose ofofatumumab did not demonstrate maternal
toxicity or teratogenicity. Ofatumumab crossed the placental barrier, and fetuses
exhibited depletion of peripheral B cells and decreased spleen and placental
weights. ARZERRA should be used during pregnancy only if the potential benefit
to the mother justifies the potential risk to the fetus. There are no human or
animal data on the potential short- and long-term effects of perinatal B-cell
depletion in offspring following in utero exposure to ofatumumab. Ofatumumab
does not bind normal human tissues other than B lymphocytes. It is not known
if binding occurs to unique embryonic or fetal tissue targets. In addition, the
kinetics of B-lymphocyte recovery are unknown in offspring with B-cell depletion
[see Nonclinical Toxicology (13.3)]. 8.3 Nursing Mothers It is not known whether
ofatumumab is secreted in human milk; however, human IgG is secreted in
human milk. Published data suggest that neonatal and infant consumption of
breast milk does not result in substantial absorption of these maternal antibodies
into circulation. Because the effects of local gastrointestinal and limited systemic
exposure to ofatumumab are unknown, caution should be exercised when
ARZERRA is administered to a nursing woman. 8.4 Pediatric Use Safety and
effectiveness of ARZERRA have not been established in children. 8.5 Geriatric
Use Clinical studies of ARZERRA did not include sufficient numbers of subjects
aged 65 and over to determine whether they respond differently from younger
subjects [see Clinical Pharmacology (12.3) of full prescribing information].
8.6 Renal Impairment No formal studies of ARZERRA in patients with renal
impairment have been conducted [see Clinical Pharmacology (12.3) of full
prescribing information]. 8.7 Hepatic Impairment No formal studies of ARZERRA
in patients with hepatic impairment have been conducted.
10 OVERDOSAGE
No data are available regarding overdosage with ARZERRA.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility No carcinogenicity
or mutagenicity studies ofofatumumab have been conducted. In a repeat-dose
toxicity study, no tumorigenic or unexpected mitogenic responses were noted in
cynomolgus monkeys treated for 7 months with up to 3.5 times the human dose
ofofatumumab. Effects on male and female fertility have not been evaluated
in animal studies. 13.3 Reproductive and Developmental Toxicology
Pregnant cynomolgus monkeys dosed with 0.7 or 3.5 times the human dose
ofofatumumab weekly during the period of organogenesis (gestation days
20 to 50) had no maternal toxicity or teratogenicity. Both dose levels of
ofatumumab depleted circulating B cells in the dams, with signs of initial
B cell recovery 50 days after the final dose. Following Caesarean section
at gestational day 100, fetuses from ofatumumab-treated dams exhibited
decreases in mean peripheral B-cell counts (decreased to approximately
10% of control values), splenic B-cell counts (decreased to approximately
15 to 20% of control values), and spleen weights (decreased by 15% for the
low-dose and by 30% for the high-dose group, compared to control values).
Fetuses from treated dams exhibiting anti-ofatumumab antibody responses
had higher B cell counts and higher spleen weights compared to the fetuses
from other treated dams, indicating partial recovery in those animals
developing anti-ofatumumab antibodies. When compared to control animals,
fetuses from treated dams in both dose groups had a 10% decrease in mean
placental weights. A 15% decrease in mean thymus weight compared to
the controls was also observed in fetuses from dams treated with 3.5 times
the human dose ofofatumumab. The biological significance of decreased
placental and thymic weights is unknown. The kinetics of B-lymphocyte
recovery and the potential long-term effects of perinatal B-cell depletion in
offspring from ofatumumab-treated dams have not been studied in animals.
17 PATIENT COUNSELING INFORMATION
Advise patients to contact a healthcare professional for any of the following:
• Signs and symptoms of infusion reactions including fever, chills, rash,
or breathing problems within 24 hours of infusion [see Warnings and
Precautions (5.1) and Adverse Reactions (6.1)]
• Bleeding, easy bruising, petechiae, pallor, worsening weakness, or fatigue
[see Warnings and Precautions (5.2)]
• Signs of infections including fever and cough [see Warnings and
Precautions (5.2) and Adverse Reactions (6.1)]
• New neurological symptoms such as confusion, dizziness or loss of
balance, difficulty talking or walking, or vision problems [see Warnings and
Precautions (5.3)]
• Symptoms of hepatitis including worsening fatigue or yellow discoloration
of skin or eyes [see Warnings and Precautions (5.4)]
• New or worsening abdominal pain or nausea [see Warnings and
Precautions (5.5)]
• Pregnancy or nursing [see Use in Specific Populations (8.1, 8.3)]
Advise patients of the need for:
• Periodic monitoring for blood counts [see Warnings and Precautions (5.2)]
• Avoiding vaccination with live viral vaccines [see Warnings and
Precautions (5.6)]
Manufactured by:
GLAXO GROUP LIMITED
Greenford, Middlesex, UB6 0NN, United Kingdom
U.S. Lic. 1809
Distributed by:
GlaxoSmithKline
Research Triangle Park, NC 27709
©2011, GlaxoSmithKline. All rights reserved.
September 2011
ARZ:6BRS
©2011 The GlaxoSmithKline Group of Companies
All rights reserved. Printed in USA. AZA293R0 September 2011
The Journal of Clinical Issues in Community Practice
Call for Papers
We are seeking papers in the following categories:
• Original Research • Reviews
• Letters • Case Letters
• Research Letters • Commentaries
To submit a paper, go to http://editorialmanager.com/co/
Editor-in-Chief
David H. Henry,
MD, FACP
Pennsylvania Hospital
Philadelphia
Editorial Board
Athanassios Argiris, MD
University of Texas Health Science Center, San Antonio
Ludovico Balducci, MD
Moffitt Cancer Center, Tampa, FL
Johanna Bendell, MD
Sarah Cannon Research Institute, Nashville, TN
Charles L. Bennett, MD, PhD, MPP
University of South Carolina, Columbia
Roy A. Beveridge, MD
US Oncology, Houston, TX
Ralph V. Boccia, MD
Georgetown University, Washington, DC
Matt Brow
US Oncology, Washington, DC
Leslie T. Busby, MD
Rocky Mountain Cancer Centers, Boulder, CO
Sant P. Chawla, MD, FRACP
Sarcoma Oncology Center Santa Monica, CA
Michael J. Fisch, MD, MPH
The University of Texas
MD Anderson Cancer Center, Houston
John A. Fracchia, MD
Lenox Hill Hospital, New York
James N. George, MD
University of Oklahoma Health Sciences Center
Oklahoma City
James Gilmore, PharmD
Georgia Cancer Specialists, Atlanta
Axel Grothey, MD
Mayo Clinic, Rochester, MN
Daniel G. Haller, MD
Professor Emeritus, University of Pennsylvania, Bryn Mawr
David M.J. Hoffman, MD
Tower Hematology Oncology Medical Group
Beverly Hills, CA
Jimmie Holland, MD
Memorial Sloan-Kettering Cancer Center, New York
Thomas Julian, MD
Allegheny General Hospital, Pittsburgh, PA
Vivek Kavadi, MD
Texas Oncology Cancer Center, Austin
Shaji Kumar, MD
Mayo Clinic, Rochester, MN
Kartik Konduri
Texas Oncology Cancer Center, Austin
Corey J Langer, MD, FACP
University of Pennsylvania, Philadelphia
Editors
Jame Abraham, MD
West Virginia University
Morgantown, WV
Linda D. Bosserman,
MD, FACP
Wilshire Oncology
Medical Group
La Verne, CA
January 2013
VOLUME 10, NUMBER 1
Debra A. Patt,
MD, MPH
Texas Oncology Cancer
Center, Austin
Stuart M. Lichtman, MD
Memorial Sloan-Kettering Cancer Center, Commack, NY
John L. Marshall, MD
Lombardi Comprehensive Cancer Center, Washington, DC
Cathy Maxwell, RN, OCN, CCRC
Advanced Medical Specialties, LLC, Miami, FL
Bradley J. Monk, MD, FACOG
Creighton University School of Medicine at St. Joseph’s Hospital and
Medical Center, Phoenix, AZ
Anne Moore, MD
Weill Medical College of Cornell University, New York
Eric Nadler, MD
Texas Oncology Cancer Center, Austin
Deborah A. Nagle, MD
Beth Israel Deaconess Medical Center, Boston, MA
Marcus Neubauer, MD
University of Kansas Cancer Center, Overland Park
Geoffrey R. Norman, PhD
McMaster University, Hamilton, Ontario, Canada
Steven O’Day, MD
The Angeles Clinic & Research Institute, Los Angeles, CA
Theodore A. Okon, MBA
Supportive Oncology Services, Memphis, TN
Philip A. Philip, MD, PhD
Barbara Ann Karmanos Cancer Institute, Detroit, MI
Jondavid Pollock, MD, PhD
Schiffler Cancer Center, Wheeling, WV
Nicholas J. Robert, MD
US Oncology, Fairfax, VA
Peter J. Rosen, MD
Roy & Patricia Disney Family
Cancer Research Center, Burbank, CA
Myrna R. Rosenfeld, MD, PhD
University of Pennsylvania School of Medicine, Philadelphia
Lee S. Schwartzberg, MD, FACP
The West Clinic, Memphis, TN
Mark A. Sitarik, MD
Rocky Mountain Cancer Centers, Boulder, CO
David Streiner, PhD, CPsych
University of Toronto, Toronto, Ontario, Canada
Debu Tripathy, MD
University of Southern California/ Norris Comprehensive Cancer Center,
Los Angeles
Steven Tucker, MD
Tucker Medical, Singapore, Malaysia
Volume 10/Number 1 January 2013 COMMUNITY ONCOLOGY A5
Information for Authors and Advertisers
Aims and Scope
COMMUNITY ONCOLOGY is an independent journal that publishes peerreviewed
research, review articles and commentary on all aspects of
clinical oncology practice. Article types include original clinical studies in
practice-based settings, state-of-the-art review papers, peer viewpoints,
commentaries, and letters to the editor.
For a full and complete guide for authors, go to ees.elsevier.com/co/
For further information, contact the Managing Editor, Renée Matthews,
at 240-221-2461 or e-mail, renee.matthews@elsevier.com.
Correspondence
For general, noneditorial enquiries, write to COMMUNITY ONCOLOGY, 7
Century Drive, Suite 302, Parsippany, NJ 07054-4609.
Letters to the Editor should be addressed to the Editor-in-Chief, David
H. Henry, MD, FACP, e-mail: c.oncology@elsevier.com.
Advertising
For information regarding advertising rates, contact Peter Murphy
(tel: 201-529-4020; e-mail: pmurphy@braveheart-group.com) or Stuart
Williams (tel: 201-529-4004; e-mail: swilliams@braveheart-group.com);
for information regarding supplements and projects, contact Devin
Gregorie (tel: 516-381-8613; e-mail: d.gregorie@elsevier.com).
CME Supplements
For information on CME supplements to COMMUNITY ONCOLOGY,
contact Sylvia Reitman of Global Academy for Medical Education,
LLC, at e-mail: s.reitman@globalacademycme.com.
Annual Subscription Rates
For 12 issues (in US$): Individual $380, Canada $413, International
$413; Institutional $380, Canada $413, International, $413; Single
copy $45.
For further information regarding subscriptions, contact Subscription
Inquiry Line 1-800-480-4851.
COMMUNITY ONCOLOGY (ISSN 1548-5315) is published monthly
by Frontline Medical Communications Inc, 7 Century Drive, Suite 302,
Parsippany, NJ 07054-4609.
January 2013
VOLUME 10, NUMBER 1
Copyright
© Copyright 2013 by Frontline Medical Communications Inc. No part
of this publication may be reproduced or transmitted in any form or by
any means, electronic or mechanical, including photocopy, recording, or
any information storage and retrieval system, without written permission
from the Publisher.
Disclaimer
Discussions, views, opinions, and recommendations as to medical
procedures, products, choice of drugs, and drug dosages are the
responsibility of the authors or advertisers. No responsibility is assumed
by the Publisher, Editor, or Editorial Board for any injury and/or
damage to persons or property as a matter of product liability,
negligence, or otherwise or from any use or operation of any methods,
products, instructions, or ideas contained in the material herein. Because
of rapid advances in the medical sciences, independent verification of
diagnoses and drug dosages should be made. Advertiser and advertising
agency recognize, accept, and assume liability for all content (including
text, representations, illustrations, opinions, and facts) of advertisements
printed and also assume responsibility for any claims made against the
Publisher arising from or related to such advertisements.
In the event that legal action or a claim is made against the Publisher
arising from or related to such advertisements, advertiser and advertising
agency agree to fully defend, indemnify, and hold harmless the Publisher
and to pay any judgment, expenses, and legal fees incurred by the
Publisher as a result of said legal action or claim. The Publisher reserves
the right to reject any advertising that he feels is not in keeping with the
publication’s standards.
The Publisher is not liable for delays in delivery and/or nondelivery in
the event of Act of God, action by any government or quasigovernmental
entity, fire, flood, insurrection, riot, explosion, embargo,
strikes (whether legal or illegal), labor or material shortage, transportation
interruption of any kind, work slowdown, or any condition beyond the
control of the Publisher that affects production or delivery in any
manner.
This journal is printed on paper meeting the requirements of ANSI/
NISO Z39.48-1992 (Permanence of Paper) effective with Volume 1,
Issue 1, 2004.
A6 COMMUNITY ONCOLOGY January 2013 www.CommunityOncology.net
Alan Imhoff, President and Publisher
Mary Jo Dales, Editorial Director
Reneé Matthews, Managing Editor
Neil Osterweil, Contributing Writer
Rebecca Slebodnik, Production Specialist
Peter Murphy, Stuart Williams, National
Accounts Managers
Devin Gregorie, National Accounts
Manager—Oncology Projects
a publication by IMNG Medical
Media, a division of Frontline
Medical Communications Inc.
COMMUNITY ONCOLOGY (ISSN 1548-5315) is
published monthly by Frontline Medical Communications
Inc, 7 Century Drive, Suite 302, Parsippany,
NJ 07054-4609. Periodicals postage paid at Parsippany,
NJ, and additional mailing offices.
Change of Address
Postmaster: send changes of address to COMMUNITY
ONCOLOGY, Subscription Service, 151 Fairchild Ave.,
Suite 2, Plainview, NY 11803-1709.
Recipient: to change your address, contact
Subscription Services 1-800-480-4851.
FROM THE EDITOR
1 From ASH, studies that advance the treatment of
hematologic malignancies
David H. Henry, MD, FACP
COMMENTARY
3 Regorafenib monotherapy for previously treated metastatic
colorectal cancer
Stuart M. Lichtman, MD
COMMUNITY TRANSLATIONS
5 Regorafenib in previously treated metastatic colorectal
cancer
Edited by Jame Abraham, MD; report prepared by Matt Stenger, MS
ORIGINALRESEARCH
January 2013
VOLUME 10, NUMBER 1
contents
8 ImpactofpretreatmentPET on disease control and
treatment decisions in locoregionally advanced esophageal
cancer patients treated with chemoradiotherapy
Patricia L. Watkins, MS, John M. Watkins, MD, A. Jason Zauls, MD,
Daniel T. Lackland, DrPH, M. Boyd Gillespie, MD, Thomas C. Hulsey, ScD,
and Joseph M. Jenrette III, MD
16 Variation by age in neutropenic complications among
patients with cancer receiving chemotherapy
Henry J. Henk, PhD, James A. Kaye, MD, DrPH, Kenneth J. Rothman, DrPH,
Laura K. Becker, MS, Jason C. Legg, PhD, Xiaoyan Li, PhD, and
Robert Deeter, PharmD
LETTERS
Case Letter
27 Plasmablastic lymphoma presenting as proptosis and
impending visual loss
Mihaela Vatca, MD, Katherine Robbins, MD, David D. Grier, MD,
James O. Cappellari IV, MD, and Seema Naik, MD
FEATURES
Technology
33 LinkedIn Facebook HIPPA friendly and secure Doximity
Neil Osterweil
Volume 10/Number 1 January 2013 COMMUNITY ONCOLOGY A7
For News and Views that
matter to supportive care
in oncology
Follow us on Twitter
@JSupportOncol
www.supportiveoncology.net
Important Safety Information
Serious adverse reactions associated with SYNRIBO
are myelosuppression (including thrombocytopenia,
neutropenia, and anemia), bleeding, and
hyperglycemia. Some adverse reactions, such as
myelosuppression, gastrointestinal hemorrhages,
and cerebral hemorrhage, have been severe and/or
fatal. Patients with neutropenia are at an increased
risk of infection
Closely monitor patient complete blood counts,
symptoms of infection or fever. Monitor glucose levels,
especially in patients with diabetes or risk factors for
diabetes. Avoid SYNRIBO in patients with poorly
controlled diabetes mellitus until good glycemic
control has been established. Consider dose
modifications for toxicities
©2012 Cephalon, Inc., a wholly owned subsidiary of Teva Pharmaceutical Industries Ltd.
All rights reserved. OMA-0027 November 2012. Printed in USA.
NOW APPROVED
SYNRIBO.com
SYNRIBO (omacetaxine mepesuccinate) for
Injection, for subcutaneous use, is indicated for
the treatment of adult patients with chronic or
accelerated phase chronic myeloid leukemia
(CML) with resistance and/or intolerance to
two or more tyrosine kinase inhibitors (TKIs).
This indication is based upon response rate.
There are no trials verifying an improvement in
disease-related symptoms or increased survival
with SYNRIBO.
SYNRIBO can cause fetal harm when administered
to a pregnant woman. Women should be advised to
avoid becoming pregnant while using SYNRIBO
Most common adverse reactions (frequency ≥20%)
in chronic and accelerated phase patients:
thrombocytopenia, anemia, neutropenia, diarrhea,
nausea, fatigue, asthenia, injection site reaction,
pyrexia, infection, and lymphopenia
Please see the following brief summary
of Prescribing Information.
BRIEF SUMMARY OF PRESCRIBING INFORMATION
See package insert for full Prescribing Information.
SYNRIBO (omacetaxine mepesuccinate) for Injection, for subcutaneous use
1 INDICATIONS AND USAGE
SYNRIBO is indicated for the treatment of adult patients with chronic or accelerated phase chronic
myeloid leukemia (CML) with resistance and/or intolerance to two or more tyrosine kinase inhibitors (TKI).
This indication is based upon response rate. There are no trials verifying an improvement in disease-related
symptoms or increased survival with SYNRIBO.
2 DOSAGE AND ADMINISTRATION
2.1 Induction Schedule
The recommended starting schedule for induction is 1.25 mg/m2 administered subcutaneously twice daily
for 14 consecutive days every 28 days, over a 28-day cycle. Cycles should be repeated every 28 days until
patients achieve a hematologic response.
2.2 Maintenance Dosing
Professional Wordpress Plugin Developmen... 4074KB Jun
 CONTENTS Foreword Introduction Chapter 1 : An Introduction to Plugins What is a Plugin? Scheduling Cron Events True Cron Practical Use Summary Chapter 14 : The Rewrite API Why Rewrite URLs How WordPress Handles Queries Practical Uses Summary Chapter 15 : Multisite Differences Enabling Multisite in WordPress Multisite Functions Multisite Database Schema 7 Summary Chapter 16 : Debugging and Optimizing Supporting Old Versions (Not) Debugging Error Logging Caching Summary Chapter 17 : Marketing Your Plugin Choosing a License for Your Plugin Submitting to WordPress.org Getting Your Plugin Renowned Summary Chapter 18 : The Developer Toolbox Core as Reference Codex Tool Web Sites Community Resources 8 Tools Summary Index 9 10 11 Professional WordPress® Plugin Development Published by Wiley Publishing, Inc. 10475 Crosspoint Boulevard Indianapolis, IN 46256 www.wiley.com Copyright © 2011 by Wiley Publishing, Inc., Indianapolis, Indiana Published simultaneously in Canada ISBN: 978-0-470-91622-3 ISBN: 978-1-118-07530-2 (ebk) ISBN: 978-1-118-07532-6 (ebk) ISBN: 978-1-118-07531-9 (ebk) No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, 12 (978) 750-8400, fax (978) 646-8600. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley.com/go/permissions. Limit of Liability/Disclaimer of Warranty: The publisher and the author make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation warranties of fitness for a particular purpose. No warranty may be created or extended by sales or promotional materials. The advice and strategies contained herein may not be suitable for every situation. This work is sold with the understanding that the publisher is not engaged in rendering legal, accounting, or other professional services. If professional assistance is required, the services of a competent professional person should be sought. Neither the publisher nor the author shall be liable for damages arising herefrom. The fact that an organization or Web site is referred to in this work as a citation and/or a potential source of further information does not mean that the author or the publisher endorses the information the organization or Web site may provide or recommendations it may make. Further, readers should be aware that Internet Web sites listed in this work may have changed or disappeared between when this work was written and when it is read. For general information on our other products and services please contact our Customer Care Department within the United States at (877) 762-2974, outside the United States at (317) 572-3993 or fax (317) 572-4002. 13 Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books. Library of Congress Control Number: 2011920897 Trademarks: Wiley, the Wiley logo, Wrox, the Wrox logo, Wrox Programmer to Programmer, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates, in the United States and other countries, and may not be used without written permission. WordPress is a registered trademark of Automattic, Inc. All other trademarks are the property of their respective owners. Wiley Publishing, Inc., is not associated with any product or vendor mentioned in this book. 14 To my Father, Robert “Basket Bob” Williams, for inspiring me to become the man I am today. — Brad Williams To my wife Ariane for her support while I was escaping household chores, and to my kids Oscar and Cyrus who’ll be WordPress hackers in 10 years. — Ozh Richard To my family for allowing me to explore the online world as a career path and the WordPress community for inviting me in. — Justin Tadlock 15 CREDITS Executive Editor Carol Long Project Editor Kelly Talbot Technical Editors Doug Vann Andrew Nacin Production Editor Rebecca Anderson Copy Editor Apostrophe Editing Services Editorial Director Robyn B. Siesky Editorial Manager Mary Beth Wakefield Production Manager 16 Tim Tate Vice President and Executive Group Publisher Richard Swadley Vice President and Executive Publisher Barry Pruett Associate Publisher Jim Minatel Project Coordinator, Cover Katie Crocker Proofreader Jen Larsen, Word One New York Indexer Johnna VanHoose Dinse Cover Designer Michael E. Trent Cover Photo © pagadesign/istockphoto.com 17 ABOUT THE AUTHORS BRAD WILLIAMS is the CEO and co-founder of WebDevStudios.com. He is also a co-host on the SitePoint podcast and the co-author of Professional WordPress. Brad has been developing websites for more than 14 years, including the last 4 where he has focused on open-source technologies like WordPress. Brad has given presentations at various WordCamps across the country, is the organizer for the New Jersey and Philadelphia WordPress Meetups and WordCamp Philly. In 2010 Brad founded Pluginize.com, a company dedicated to building custom WordPress plugins. OZH RICHARD is a web developer who started to use WordPress at version 1.0.1, published his first WordPress-powered website in May 2004, and released his first plugin three months later. He has since developed several popular plugins, won an Annual WordPress Plugin Competition, and is now an official judge. When not coding WordPress plugins or sharing tutorials, Ozh contributes to other Open Source projects such as YOURLS, a self-hosted URL shortener, or plays Quake. You can find Ozh online at http://ozh.org/. JUSTIN TADLOCK is a Web developer and designer who coded his first Web page in 2003 at the age of 18, only months after getting his first computer. He found WordPress in 2005 and has been working with and contributing to the platform ever since. He has developed many popular WordPress plugins and themes while exploring several business paths using the open-source platform. 18 ACKNOWLEDGMENTS THANK YOU to the love of my life, April, for your endless support, friendship, and continuing to put up with my nerdy ways. Thank you to my awesome nieces, Indiana Brooke and Austin Margaret. Thank you Carol Long for believing in this book idea and helping make it a reality. To Ozh and Justin, two amazing co-authors, your knowledge of WordPress is unmatched, and this book wouldn’t have been what it is without you both. Thank you to the entire WordPress community for your support, friendships, motivation, and guidance. Thank you fizzypop for making WordCamp after parties the stuff of legend. Last but not least thank you to my ridiculous zoo: Lecter, Clarice, and Squeaks the Cat (aka Kitty Galore). Your smiling faces and wiggly butts always put a smile on my face. — Brad Williams IT’S BEEN A LONG TIME in the WordPress community since I first started to dissect the few plugins that began to pop like daisies in 2004 and tried to understand how things worked. To all the coders who released the code that taught me the innards of WordPress, I can’t express how much I owe you. To all the members of the WordPress community who don’t write code but foster the creativity and water our community, thank you for your invaluable dedication. To Brad, who sent me that crazy proposal about a plugin book, I hope I’ll cross the oceans one day to have a few beers with you. To Ronnie James Dio, Tom Araya, Bruce Dickinson, Blaze Bayley, Lemmy Kilmister, Dave Mustaine, Rob Zombie, Till Lindemann, and Mike Muir, whose gentle 19 voices have lulled me and inspired me while I was writing late at night. — Ozh Richard THE WORDPRESS COMMUNITY took me in as a lost kid who was trying to figure out life and presented me with opportunities that I’d never dreamed possible. A simple “thank you” is an understatement. To my plugin and theme users, you continue to inspire me and keep my skills sharp with your invaluable feedback and loyalty. To Brad, thank you for that oddly random email about writing a plugin book. To Ozh, thank you for coding all those cool plugins I learned from before becoming a developer myself. To Granny, thank you for allowing me to skip several dinners to work on this book. To my family and friends, thank you for supporting me and showing superhuman patience during hour-long conversations (i.e., crazed rants) about plugin development. Most importantly, to my father, who knows nothing about Web development but taught me everything about being successful and continues to teach me today. — Justin Tadlock 20 FOREWORD STARTING OUT as a simple blogging system, over the last few years WordPress has morphed into a fully featured and widely used content management system. It offers individuals and companies world-wide a free and open-source alternative to closed-source and often very expensive systems. When I say fully featured, that’s really only true because of the ability to add any functionality needed in the form of a plugin. The core of WordPress is simple: You add in functionality with plugins as you need it. Developing plugins allows you to stand on the shoulders of a giant: You can showcase your specific area of expertise and help users benefit while not having to deal with parts of WordPress you don’t care or know about. I’ve written dozens of plugins, which together have been downloaded millions of times. Doing that has changed my life. It has helped me build out a business for myself, doing development and (SEO) consultancy work. This is in your outreach too! I wish that when I started developing plugins for WordPress as a hobby, some five years back, this book had been around. It would have saved me countless hours of digging through code and half-finished documentation. I always ended up redoing pieces because I’d found yet another best practice or simply an easier way of doing things. Although this book didn’t exist yet, the authors of this book have always been a source of good information for me while 21 developing my plugins. Each of them is an expert in his own right; together they are one of the best teams that could have been gathered to write this book. WordPress makes it easy for people to have their say through words, sound, and visuals. For those who write code, WordPress allows you to express yourself in code. And it’s simple. Anyone can write a WordPress plugin. With this guide in hand, you can write a plugin that is true to WordPress’ original vision: Code is Poetry. Happy coding! Joost de Valk Yoast.com 22 INTRODUCTION DEAR READER, thank you for picking up this book! You have probably heard about WordPress already, the most popular self-hosted content management system (CMS) and blogging software in use today. WordPress powers literally millions of Web sites on the Internet, including high profile sites such as TechCrunch and CNN’s blog. What makes WordPress so popular is that it’s free, open source, and extendable beyond limits. Thanks to a powerful, architecturally sound, and easy-to-use plugin system, you can customize how WordPress works and extend its functionalities. There are already more than ten thousand plugins freely available in the official plugin repository, but they won’t suit all your needs or client requests. That’s where this book comes in handy! As of this writing, we (Brad, Ozh, and Justin), have publicly released 50 plugins, which have been downloaded nearly one million times, and that’s not counting private client work. This is a precious combined experience that we are going to leverage to teach you how to code your own plugins for WordPress by taking a hands-on approach with practical examples and real life situations you will encounter with your clients. The primary reason we wanted to write this book is to create a preeminent resource for WordPress plugin developers. When creating plugins for WordPress, it can be a challenge to find the resources needed in a single place. Many of the online tutorials and guides are outdated and recommend incorrect methods for plugin development. This book is one of the most 23 extensive collections of plugin development information to date and should be considered required reading for anyone wanting to explore WordPress plugin development from the ground up. WHO THIS BOOK IS FOR This book is for professional Web developers who want to make WordPress work exactly how they and their clients want. WordPress has already proven an exceptional platform for building any type of site from simple static pages to networks of full-featured communities. Learning how to code plugins will help you get the most out of WordPress and have a cost-effective approach to developing per-client features. This book is also for the code freelancers who want to broaden their skill portfolio, understand the inner workings of WordPress functionality, and take on WordPress gigs. Since WordPress is the most popular software to code and power websites, it is crucial that you understand how things run under the hood and how you can make the engine work your way. Learning how to code plugins will be a priceless asset to add to your resume and business card. Finally, this book is for hobbyist PHP programmers who want to tinker with how their WordPress blog works, discover the infinite potential of lean and flexible source code, and how they can interact with the flow of events. The beauty of open source is that it’s easy to learn from and easy to give back in turn. This book will help you take your first step into a community that will welcome your creativity and contribution. 24 Simply put, this book is for anyone who wants to extend the way WordPress works, whether it is for fun or profit. WHAT YOU NEED TO USE THIS BOOK This book assumes you already have a Web server and WordPress running. For your convenience it is preferred that your Web server runs on your localhost, as it will be easier to modify plugin files as you read through the book, but an online server is also fine. Code snippets written in PHP are the backbone of this book: You should be comfortable with reading and writing basic PHP code or referring to PHP’s documentation to fill any gaps in knowledge about fundamental functions. Advanced PHP code tricks are explained, so you don’t need to be a PHP expert. You will need to have rudimentary HTML knowledge to fully understand all the code. A basic acquaintance with database and MySQL syntax will help with grasping advanced subjects. To make the most of the chapter dedicated to JavaScript and AJAX, comprehension of JavaScript code and jQuery syntax will be a plus. WHAT THIS BOOK COVERS As of this writing, WordPress 3.1 is around the corner and this book has been developed alongside this version. Following the best coding practices outlined in this book and using built-in APIs are keys to future-proof code that will not be deprecated when a newer version of WordPress is released. We believe that every code snippet in this book will still be 25 accurate and up-to-date for several years, just as several plugins we coded many years ago are still completely functional today. HOW THIS BOOK IS STRUCTURED This book is, to date, one of the most powerful and comprehensive resources you can find about WordPress plugins. Advanced areas of the many WordPress APIs are covered, such as the Rewrite APIs, cron jobs, and Custom Post Types. This book is divided into three major parts. Reading the first three chapters (Introduction, Plugin Foundations, and Hooks) is required if you are taking your first steps in the wonders of WordPress plugins. Chapters 4 through 7 will cover most common topics in coding plugins, and understanding them will be useful when reading subsequent chapters. The remaining chapters cover advanced APIs and functions, can be read in any order, and will sometimes refer to other chapters for details on a particular function. CONVENTIONS To help you get the most from the text and keep track of what’s happening, we’ve used a number of conventions throughout the book. Boxes with a warning icon like this one hold important, not-to-be-forgotten information that is directly relevant to the surrounding text. 26 The pencil icon indicates notes, tips, hints, tricks, and asides to the current discussion. As for styles in the text: • We highlight new terms and important words when we introduce them. • We show keyboard strokes like this: Ctrl+A. • We show file names, URLs, and code within the text like so: persistence.properties. • We present code in two different ways: We use a monofont type with no highlighting for most code examples. We use bold to emphasize code that is particularly important in the present context or to show changes from a previous code snippet. SOURCE CODE As you work through the examples in this book, you may choose either to type in all the code manually, or to use the source code files that accompany the book. All the source code used in this book is available for download at www.wrox.com. When at the site, simply locate the book’s title (use the Search box or one of the title lists) and click the Download Code link on the book’s detail page to obtain all the source code for the book. Code that is included on the Web site is highlighted by the following icon: 27 Listings include the filename in the title. If it is just a code snippet, you’ll find the filename in a code note such as this: Code snippet filename Because many books have similar titles, you may find it easiest to search by ISBN; this book’s ISBN is 978-0-470-91622-3. Once you download the code, just decompress it with your favorite compression tool. Alternately, you can go to the main Wrox code download page at www.wrox.com/dynamic/ books/download.aspx to see the code available for this book and all other Wrox books. ERRATA We make every effort to ensure that there are no errors in the text or in the code. However, no one is perfect, and mistakes do occur. If you find an error in one of our books, like a spelling mistake or faulty piece of code, we would be very grateful for your feedback. By sending in errata, you may save another reader hours of frustration, and at the same time, you will be helping us provide even higher quality information. 28 To find the errata page for this book, go to www.wrox.com and locate the title using the Search box or one of the title lists. Then, on the book details page, click the Book Errata link. On this page, you can view all errata that has been submitted for this book and posted by Wrox editors. A complete book list, including links to each book’s errata, is also available at www.wrox.com/misc-pages/booklist.shtml. If you don’t spot “your” error on the Book Errata page, go to www.wrox.com/contact/techsupport.shtml and complete the form there to send us the error you have found. We’ll check the information and, if appropriate, post a message to the book’s errata page and fix the problem in subsequent editions of the book. P2P.WROX.COM For author and peer discussion, join the P2P forums at p2p.wrox.com. The forums are a Web-based system for you to post messages relating to Wrox books and related technologies and interact with other readers and technology users. The forums offer a subscription feature to email you topics of interest of your choosing when new posts are made to the forums. Wrox authors, editors, other industry experts, and your fellow readers are present on these forums. At p2p.wrox.com, you will find a number of different forums that will help you, not only as you read this book, but also as you develop your own applications. To join the forums, just follow these steps: 1. Go to p2p.wrox.com and click the Register link. 29 2. Read the terms of use and click Agree. 3. Complete the required information to join, as well as any optional information you wish to provide, and click Submit. 4. You will receive an email with information describing how to verify your account and complete the joining process. You can read messages in the forums without joining P2P, but in order to post your own messages, you must join. Once you join, you can post new messages and respond to messages other users post. You can read messages at any time on the Web. If you would like to have new messages from a particular forum emailed to you, click the Subscribe to this Forum icon by the forum name in the forum listing. For more information about how to use the Wrox P2P, be sure to read the P2P FAQs for answers to questions about how the forum software works, as well as many common questions specific to P2P and Wrox books. To read the FAQs, click the FAQ link on any P2P page. 30 Chapter 1 An Introduction to Plugins WHAT’S IN THIS CHAPTER? • • • • • Understanding a plugin Using available WordPress APIs Loading order of plugins Finding examples of popular plugins Determining the separation of plugin and theme functionality • Managing and installing plugins • Understanding types of WordPress plugins WordPress is one of the most popular open source content management systems available today. One of the primary reasons WordPress is so popular is the ease with which you can customize WordPress through plugins. WordPress has an amazing framework in place giving plugin developers the tools needed to extend WordPress in any way imaginable. Understanding how plugins work, and the tools available in WordPress, is critical knowledge when developing professional WordPress plugins. WHAT IS A PLUGIN? A plugin in WordPress is a PHP script that extends or alters the core functionality of WordPress. Quite simply plugins are files installed in WordPress to add a feature, or set of features, to WordPress. Plugins can range in complexity from a simple social networking plugin to an extremely elaborate 31 e-commerce package. There is no limit to what a plugin can do in WordPress; because of this there is no shortage of plugins available for download. How Plugins Interact with WordPress WordPress features many different APIs for use in your plugin. Each API, or application programming interface, helps interact with WordPress in a different way. Following is a list of the main available APIs in WordPress and their function: • Plugin — Provides a set of hooks that enable plugins access to specific parts of WordPress. WordPress contains two different types of hooks: Actions and Filters. The Action hook enables you to trigger custom plugin code at specific points during execution. For example, you can trigger a custom function to run after a user registers a user account in WordPress. The Filter hook to modifies text before adding or after retrieving from the database. • Widgets — Create and manage widgets in your plugin. Widgets appear under the Appearance ⇒ Widgets screen and are available to add to any registered sidebar in your theme. The API enables multiple instances of the same widget to be used throughout your sidebars. • Shortcode — Adds shortcode support to your plugin. A shortcode is a simple hook that enables you to call a PHP function by adding something such as [shortcode] to a post or page. • HTTP — Sends HTTP requests from your plugin. This API retrieves content from an external URL or 32 • • • • • • for submitting content to a URL. Currently you have five different ways to send an HTTP request. This API standardizes that process and tests each method prior to executing. Based on your server configuration, the API will use the appropriate method and make the request. Settings — Inserts settings or a settings section for your plugin. The primary advantage to using the Settings API is security. All settings data is scrubbed, so you do not need to worry about cross site request forgery (CSRF) and cross site scripting (XSS) attacks when saving plugin settings. Options — Stores and retrieves options in your plugin. This API features the capability to create new options, update existing options, delete options, and retrieve any option already defined. Dashboard Widgets — Creates admin dashboard widgets. Widgets automatically appear on the Dashboard of WordPress and contain all standard customization features including minimize, drag/ drop, and screen options for hiding. Rewrite — Creates custom rewrite rules in your plugin. This API enables you to add static end-points (/custom-page/), structure tags (%postname%), and add additional feed links (/feed/json/). Transients — Creates temporary options (cached data) in your plugins. This API is similar to the Options API, but all options are saved with an expiration time. Database — Accesses the WordPress database. This includes creating, updating, deleting, and retrieving database records for use in your plugins. 33 WordPress also features pluggable functions. These functions enable you to override specific core functions in a plugin. For example, the wp_mail() function is a pluggable function. You can easily define this function in your plugin and send email using SMTP rather than the default method. All pluggable functions are defined in the /wp-includes/pluggable.php Core WordPress file. You can use some predefined functions during specific plugin tasks, such as when a plugin is activated or deactivated and even when a plugin is uninstalled. Chapter 2, “Plugin Foundation,” covers these functions in detail. When Are Plugins Loaded? Plugins are loaded early in the process when a WordPress powered web page is called. Figure 1-1 shows a diagram of the standard loading process when loading a page in WordPress: FIGURE 1-1 34 Figure 1-1 illustrates the standard process when loading a page in WordPress. The flow changes slightly when loading an admin page. The differences are minor and primarily concern what theme is loaded: admin theme versus your web site theme. AVAILABLE PLUGINS When researching available plugins you need to know where to find WordPress plugins. You can download plugins anywhere on the Internet, but this isn’t always a good idea. As with any software, downloading plugins from an untrusted source could lead to malware injected and compromised plugin files. It’s best to download plugins only from trusted web sites and official sources such as the official Plugin Directory. 35 Official Plugin Directory The first place to start when researching available WordPress plugins is the official Plugin Directory at WordPress.org. The Plugin Directory is located at http://wordpress.org/extend/ plugins/. With more than 10,000 plugins available and well over 100 million plugin downloads, it’s easy to see the vital role plugins play in every WordPress web site. All plugins available in the Plugin Directory are 100% GPL and free to use for personal or commercial use. Popular Plugin Examples Take a look at the five most downloaded WordPress plugins available to get a sense of their diversity: • All in One SEO Pack — Adds advanced search engine optimization functionality to WordPress. Features include custom meta data for all content, canonical URLs, custom post type support, and more! • http://wordpress.org/extend/plugins/ all-in-one-seo-pack/ • Google XML Sitemaps — Generates an XML sitemap of all content for submission to the popular search engines such as Google, Bing, and Ask.com. • http://wordpress.org/extend/plugins/ google-sitemap-generator/ • Akismet — A popular comment spam filter for WordPress. Checks all comments against the Akismet web service to verify whether the comment is spam. • http://wordpress.org/extend/plugins/akismet/ 36 • NextGEN Gallery — Adds advanced image gallery support to WordPress. You can easily create and manage image galleries and slideshows. Galleries can be embedded in posts or pages. • http://wordpress.org/extend/plugins/ nextgen-gallery/ • Contact Form 7 — Adds a contact form to any post or page in WordPress. • http://wordpress.org/extend/plugins/ contact-form-7/ As you can see, the preceding plugins can handle any task. The features added by these plugins are universal and features that most web sites on the Internet should have. Popular Plugin Tags Now you will look at some popular tags for plugins. Plugin tags are just like blog post tags, simple keywords that describe a plugin in the Plugin Directory. This makes it easy to search for existing plugins by tag. Following are popular examples: • Twitter — Everyone loves Twitter for micro-blogging and sharing links. You can find an abundance of Twitter-related plugins for WordPress. • http://wordpress.org/extend/plugins/tags/ twitter • Google — With so many different services and APIs, Google is a popular plugin tag. Everything from Google ads to Google maps have been integrated into a WordPress plugin. 37 • http://wordpress.org/extend/plugins/tags/ google • Widget — Most plugins that include a widget also use the widget tag. This is great for viewing the many different types of widgets available for WordPress. • http://wordpress.org/extend/plugins/tags/ widget Viewing popular plugin tags is a great way to get inspiration when developing new plugins for WordPress. ADVANTAGES OF PLUGINS WordPress offers many advantages to using plugins. You need to understand the advantages to building plugins to truly understand why you should build plugins. This can also help when determining the need for a specific plugin in WordPress. Not Modifying Core One of the main advantages to plugins is the ability to modify the behavior of WordPress without modifying any core files. Core files refer to any file that is a part of the default WordPress installation. Hacking core files can make it difficult to update WordPress when a new version is released. If you made any modifications to a core file, that modification would be overwritten when the update occurs. Keeping WordPress up to date with the latest version is essential in keeping your web site secure. 38 Modifying core files can also lead to an unstable web site. Different areas of WordPress rely on other areas to function as expected. If you modify a core file and it no longer works as expected, it can cause instability and quite possibly break a completely unrelated feature in WordPress. Why Reinvent the Wheel Another advantage to building plugins is the structure that already exists for your plugin. Many of the common features have already been developed and are ready for use in your plugin. For example, you can take advantage of the built-in user roles in WordPress. Using the user roles you can easily restrict your code to execute only if a user is an administrator. Look at an example: <?php if ( current_user_can( 'manage_options' ) ) { //any code entered here will only be executed IF //user is an administrator } ?> As you can see it’s easy to verify a user has proper permissions prior to executing any code in your plugin. You learn about user accounts and roles in Chapter 8, “Users.” 39 As another example, look at sending an email in WordPress. Sure you could create a new function in your plugin to send email, but why? WordPress has a handy function called wp_mail() for sending email. Look at an example: <?php $email_to = '[email protected]
CONTENTS Foreword Introduction Chapter 1 : An Introduction to Plugins What is a Plugin? Scheduling Cron Events True Cron Practical Use Summary Chapter 14 : The Rewrite API Why Rewrite URLs How WordPress Handles Queries Practical Uses Summary Chapter 15 : Multisite Differences Enabling Multisite in WordPress Multisite Functions Multisite Database Schema 7 Summary Chapter 16 : Debugging and Optimizing Supporting Old Versions (Not) Debugging Error Logging Caching Summary Chapter 17 : Marketing Your Plugin Choosing a License for Your Plugin Submitting to WordPress.org Getting Your Plugin Renowned Summary Chapter 18 : The Developer Toolbox Core as Reference Codex Tool Web Sites Community Resources 8 Tools Summary Index 9 10 11 Professional WordPress® Plugin Development Published by Wiley Publishing, Inc. 10475 Crosspoint Boulevard Indianapolis, IN 46256 www.wiley.com Copyright © 2011 by Wiley Publishing, Inc., Indianapolis, Indiana Published simultaneously in Canada ISBN: 978-0-470-91622-3 ISBN: 978-1-118-07530-2 (ebk) ISBN: 978-1-118-07532-6 (ebk) ISBN: 978-1-118-07531-9 (ebk) No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, 12 (978) 750-8400, fax (978) 646-8600. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley.com/go/permissions. Limit of Liability/Disclaimer of Warranty: The publisher and the author make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation warranties of fitness for a particular purpose. No warranty may be created or extended by sales or promotional materials. The advice and strategies contained herein may not be suitable for every situation. This work is sold with the understanding that the publisher is not engaged in rendering legal, accounting, or other professional services. If professional assistance is required, the services of a competent professional person should be sought. Neither the publisher nor the author shall be liable for damages arising herefrom. The fact that an organization or Web site is referred to in this work as a citation and/or a potential source of further information does not mean that the author or the publisher endorses the information the organization or Web site may provide or recommendations it may make. Further, readers should be aware that Internet Web sites listed in this work may have changed or disappeared between when this work was written and when it is read. For general information on our other products and services please contact our Customer Care Department within the United States at (877) 762-2974, outside the United States at (317) 572-3993 or fax (317) 572-4002. 13 Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books. Library of Congress Control Number: 2011920897 Trademarks: Wiley, the Wiley logo, Wrox, the Wrox logo, Wrox Programmer to Programmer, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates, in the United States and other countries, and may not be used without written permission. WordPress is a registered trademark of Automattic, Inc. All other trademarks are the property of their respective owners. Wiley Publishing, Inc., is not associated with any product or vendor mentioned in this book. 14 To my Father, Robert “Basket Bob” Williams, for inspiring me to become the man I am today. — Brad Williams To my wife Ariane for her support while I was escaping household chores, and to my kids Oscar and Cyrus who’ll be WordPress hackers in 10 years. — Ozh Richard To my family for allowing me to explore the online world as a career path and the WordPress community for inviting me in. — Justin Tadlock 15 CREDITS Executive Editor Carol Long Project Editor Kelly Talbot Technical Editors Doug Vann Andrew Nacin Production Editor Rebecca Anderson Copy Editor Apostrophe Editing Services Editorial Director Robyn B. Siesky Editorial Manager Mary Beth Wakefield Production Manager 16 Tim Tate Vice President and Executive Group Publisher Richard Swadley Vice President and Executive Publisher Barry Pruett Associate Publisher Jim Minatel Project Coordinator, Cover Katie Crocker Proofreader Jen Larsen, Word One New York Indexer Johnna VanHoose Dinse Cover Designer Michael E. Trent Cover Photo © pagadesign/istockphoto.com 17 ABOUT THE AUTHORS BRAD WILLIAMS is the CEO and co-founder of WebDevStudios.com. He is also a co-host on the SitePoint podcast and the co-author of Professional WordPress. Brad has been developing websites for more than 14 years, including the last 4 where he has focused on open-source technologies like WordPress. Brad has given presentations at various WordCamps across the country, is the organizer for the New Jersey and Philadelphia WordPress Meetups and WordCamp Philly. In 2010 Brad founded Pluginize.com, a company dedicated to building custom WordPress plugins. OZH RICHARD is a web developer who started to use WordPress at version 1.0.1, published his first WordPress-powered website in May 2004, and released his first plugin three months later. He has since developed several popular plugins, won an Annual WordPress Plugin Competition, and is now an official judge. When not coding WordPress plugins or sharing tutorials, Ozh contributes to other Open Source projects such as YOURLS, a self-hosted URL shortener, or plays Quake. You can find Ozh online at http://ozh.org/. JUSTIN TADLOCK is a Web developer and designer who coded his first Web page in 2003 at the age of 18, only months after getting his first computer. He found WordPress in 2005 and has been working with and contributing to the platform ever since. He has developed many popular WordPress plugins and themes while exploring several business paths using the open-source platform. 18 ACKNOWLEDGMENTS THANK YOU to the love of my life, April, for your endless support, friendship, and continuing to put up with my nerdy ways. Thank you to my awesome nieces, Indiana Brooke and Austin Margaret. Thank you Carol Long for believing in this book idea and helping make it a reality. To Ozh and Justin, two amazing co-authors, your knowledge of WordPress is unmatched, and this book wouldn’t have been what it is without you both. Thank you to the entire WordPress community for your support, friendships, motivation, and guidance. Thank you fizzypop for making WordCamp after parties the stuff of legend. Last but not least thank you to my ridiculous zoo: Lecter, Clarice, and Squeaks the Cat (aka Kitty Galore). Your smiling faces and wiggly butts always put a smile on my face. — Brad Williams IT’S BEEN A LONG TIME in the WordPress community since I first started to dissect the few plugins that began to pop like daisies in 2004 and tried to understand how things worked. To all the coders who released the code that taught me the innards of WordPress, I can’t express how much I owe you. To all the members of the WordPress community who don’t write code but foster the creativity and water our community, thank you for your invaluable dedication. To Brad, who sent me that crazy proposal about a plugin book, I hope I’ll cross the oceans one day to have a few beers with you. To Ronnie James Dio, Tom Araya, Bruce Dickinson, Blaze Bayley, Lemmy Kilmister, Dave Mustaine, Rob Zombie, Till Lindemann, and Mike Muir, whose gentle 19 voices have lulled me and inspired me while I was writing late at night. — Ozh Richard THE WORDPRESS COMMUNITY took me in as a lost kid who was trying to figure out life and presented me with opportunities that I’d never dreamed possible. A simple “thank you” is an understatement. To my plugin and theme users, you continue to inspire me and keep my skills sharp with your invaluable feedback and loyalty. To Brad, thank you for that oddly random email about writing a plugin book. To Ozh, thank you for coding all those cool plugins I learned from before becoming a developer myself. To Granny, thank you for allowing me to skip several dinners to work on this book. To my family and friends, thank you for supporting me and showing superhuman patience during hour-long conversations (i.e., crazed rants) about plugin development. Most importantly, to my father, who knows nothing about Web development but taught me everything about being successful and continues to teach me today. — Justin Tadlock 20 FOREWORD STARTING OUT as a simple blogging system, over the last few years WordPress has morphed into a fully featured and widely used content management system. It offers individuals and companies world-wide a free and open-source alternative to closed-source and often very expensive systems. When I say fully featured, that’s really only true because of the ability to add any functionality needed in the form of a plugin. The core of WordPress is simple: You add in functionality with plugins as you need it. Developing plugins allows you to stand on the shoulders of a giant: You can showcase your specific area of expertise and help users benefit while not having to deal with parts of WordPress you don’t care or know about. I’ve written dozens of plugins, which together have been downloaded millions of times. Doing that has changed my life. It has helped me build out a business for myself, doing development and (SEO) consultancy work. This is in your outreach too! I wish that when I started developing plugins for WordPress as a hobby, some five years back, this book had been around. It would have saved me countless hours of digging through code and half-finished documentation. I always ended up redoing pieces because I’d found yet another best practice or simply an easier way of doing things. Although this book didn’t exist yet, the authors of this book have always been a source of good information for me while 21 developing my plugins. Each of them is an expert in his own right; together they are one of the best teams that could have been gathered to write this book. WordPress makes it easy for people to have their say through words, sound, and visuals. For those who write code, WordPress allows you to express yourself in code. And it’s simple. Anyone can write a WordPress plugin. With this guide in hand, you can write a plugin that is true to WordPress’ original vision: Code is Poetry. Happy coding! Joost de Valk Yoast.com 22 INTRODUCTION DEAR READER, thank you for picking up this book! You have probably heard about WordPress already, the most popular self-hosted content management system (CMS) and blogging software in use today. WordPress powers literally millions of Web sites on the Internet, including high profile sites such as TechCrunch and CNN’s blog. What makes WordPress so popular is that it’s free, open source, and extendable beyond limits. Thanks to a powerful, architecturally sound, and easy-to-use plugin system, you can customize how WordPress works and extend its functionalities. There are already more than ten thousand plugins freely available in the official plugin repository, but they won’t suit all your needs or client requests. That’s where this book comes in handy! As of this writing, we (Brad, Ozh, and Justin), have publicly released 50 plugins, which have been downloaded nearly one million times, and that’s not counting private client work. This is a precious combined experience that we are going to leverage to teach you how to code your own plugins for WordPress by taking a hands-on approach with practical examples and real life situations you will encounter with your clients. The primary reason we wanted to write this book is to create a preeminent resource for WordPress plugin developers. When creating plugins for WordPress, it can be a challenge to find the resources needed in a single place. Many of the online tutorials and guides are outdated and recommend incorrect methods for plugin development. This book is one of the most 23 extensive collections of plugin development information to date and should be considered required reading for anyone wanting to explore WordPress plugin development from the ground up. WHO THIS BOOK IS FOR This book is for professional Web developers who want to make WordPress work exactly how they and their clients want. WordPress has already proven an exceptional platform for building any type of site from simple static pages to networks of full-featured communities. Learning how to code plugins will help you get the most out of WordPress and have a cost-effective approach to developing per-client features. This book is also for the code freelancers who want to broaden their skill portfolio, understand the inner workings of WordPress functionality, and take on WordPress gigs. Since WordPress is the most popular software to code and power websites, it is crucial that you understand how things run under the hood and how you can make the engine work your way. Learning how to code plugins will be a priceless asset to add to your resume and business card. Finally, this book is for hobbyist PHP programmers who want to tinker with how their WordPress blog works, discover the infinite potential of lean and flexible source code, and how they can interact with the flow of events. The beauty of open source is that it’s easy to learn from and easy to give back in turn. This book will help you take your first step into a community that will welcome your creativity and contribution. 24 Simply put, this book is for anyone who wants to extend the way WordPress works, whether it is for fun or profit. WHAT YOU NEED TO USE THIS BOOK This book assumes you already have a Web server and WordPress running. For your convenience it is preferred that your Web server runs on your localhost, as it will be easier to modify plugin files as you read through the book, but an online server is also fine. Code snippets written in PHP are the backbone of this book: You should be comfortable with reading and writing basic PHP code or referring to PHP’s documentation to fill any gaps in knowledge about fundamental functions. Advanced PHP code tricks are explained, so you don’t need to be a PHP expert. You will need to have rudimentary HTML knowledge to fully understand all the code. A basic acquaintance with database and MySQL syntax will help with grasping advanced subjects. To make the most of the chapter dedicated to JavaScript and AJAX, comprehension of JavaScript code and jQuery syntax will be a plus. WHAT THIS BOOK COVERS As of this writing, WordPress 3.1 is around the corner and this book has been developed alongside this version. Following the best coding practices outlined in this book and using built-in APIs are keys to future-proof code that will not be deprecated when a newer version of WordPress is released. We believe that every code snippet in this book will still be 25 accurate and up-to-date for several years, just as several plugins we coded many years ago are still completely functional today. HOW THIS BOOK IS STRUCTURED This book is, to date, one of the most powerful and comprehensive resources you can find about WordPress plugins. Advanced areas of the many WordPress APIs are covered, such as the Rewrite APIs, cron jobs, and Custom Post Types. This book is divided into three major parts. Reading the first three chapters (Introduction, Plugin Foundations, and Hooks) is required if you are taking your first steps in the wonders of WordPress plugins. Chapters 4 through 7 will cover most common topics in coding plugins, and understanding them will be useful when reading subsequent chapters. The remaining chapters cover advanced APIs and functions, can be read in any order, and will sometimes refer to other chapters for details on a particular function. CONVENTIONS To help you get the most from the text and keep track of what’s happening, we’ve used a number of conventions throughout the book. Boxes with a warning icon like this one hold important, not-to-be-forgotten information that is directly relevant to the surrounding text. 26 The pencil icon indicates notes, tips, hints, tricks, and asides to the current discussion. As for styles in the text: • We highlight new terms and important words when we introduce them. • We show keyboard strokes like this: Ctrl+A. • We show file names, URLs, and code within the text like so: persistence.properties. • We present code in two different ways: We use a monofont type with no highlighting for most code examples. We use bold to emphasize code that is particularly important in the present context or to show changes from a previous code snippet. SOURCE CODE As you work through the examples in this book, you may choose either to type in all the code manually, or to use the source code files that accompany the book. All the source code used in this book is available for download at www.wrox.com. When at the site, simply locate the book’s title (use the Search box or one of the title lists) and click the Download Code link on the book’s detail page to obtain all the source code for the book. Code that is included on the Web site is highlighted by the following icon: 27 Listings include the filename in the title. If it is just a code snippet, you’ll find the filename in a code note such as this: Code snippet filename Because many books have similar titles, you may find it easiest to search by ISBN; this book’s ISBN is 978-0-470-91622-3. Once you download the code, just decompress it with your favorite compression tool. Alternately, you can go to the main Wrox code download page at www.wrox.com/dynamic/ books/download.aspx to see the code available for this book and all other Wrox books. ERRATA We make every effort to ensure that there are no errors in the text or in the code. However, no one is perfect, and mistakes do occur. If you find an error in one of our books, like a spelling mistake or faulty piece of code, we would be very grateful for your feedback. By sending in errata, you may save another reader hours of frustration, and at the same time, you will be helping us provide even higher quality information. 28 To find the errata page for this book, go to www.wrox.com and locate the title using the Search box or one of the title lists. Then, on the book details page, click the Book Errata link. On this page, you can view all errata that has been submitted for this book and posted by Wrox editors. A complete book list, including links to each book’s errata, is also available at www.wrox.com/misc-pages/booklist.shtml. If you don’t spot “your” error on the Book Errata page, go to www.wrox.com/contact/techsupport.shtml and complete the form there to send us the error you have found. We’ll check the information and, if appropriate, post a message to the book’s errata page and fix the problem in subsequent editions of the book. P2P.WROX.COM For author and peer discussion, join the P2P forums at p2p.wrox.com. The forums are a Web-based system for you to post messages relating to Wrox books and related technologies and interact with other readers and technology users. The forums offer a subscription feature to email you topics of interest of your choosing when new posts are made to the forums. Wrox authors, editors, other industry experts, and your fellow readers are present on these forums. At p2p.wrox.com, you will find a number of different forums that will help you, not only as you read this book, but also as you develop your own applications. To join the forums, just follow these steps: 1. Go to p2p.wrox.com and click the Register link. 29 2. Read the terms of use and click Agree. 3. Complete the required information to join, as well as any optional information you wish to provide, and click Submit. 4. You will receive an email with information describing how to verify your account and complete the joining process. You can read messages in the forums without joining P2P, but in order to post your own messages, you must join. Once you join, you can post new messages and respond to messages other users post. You can read messages at any time on the Web. If you would like to have new messages from a particular forum emailed to you, click the Subscribe to this Forum icon by the forum name in the forum listing. For more information about how to use the Wrox P2P, be sure to read the P2P FAQs for answers to questions about how the forum software works, as well as many common questions specific to P2P and Wrox books. To read the FAQs, click the FAQ link on any P2P page. 30 Chapter 1 An Introduction to Plugins WHAT’S IN THIS CHAPTER? • • • • • Understanding a plugin Using available WordPress APIs Loading order of plugins Finding examples of popular plugins Determining the separation of plugin and theme functionality • Managing and installing plugins • Understanding types of WordPress plugins WordPress is one of the most popular open source content management systems available today. One of the primary reasons WordPress is so popular is the ease with which you can customize WordPress through plugins. WordPress has an amazing framework in place giving plugin developers the tools needed to extend WordPress in any way imaginable. Understanding how plugins work, and the tools available in WordPress, is critical knowledge when developing professional WordPress plugins. WHAT IS A PLUGIN? A plugin in WordPress is a PHP script that extends or alters the core functionality of WordPress. Quite simply plugins are files installed in WordPress to add a feature, or set of features, to WordPress. Plugins can range in complexity from a simple social networking plugin to an extremely elaborate 31 e-commerce package. There is no limit to what a plugin can do in WordPress; because of this there is no shortage of plugins available for download. How Plugins Interact with WordPress WordPress features many different APIs for use in your plugin. Each API, or application programming interface, helps interact with WordPress in a different way. Following is a list of the main available APIs in WordPress and their function: • Plugin — Provides a set of hooks that enable plugins access to specific parts of WordPress. WordPress contains two different types of hooks: Actions and Filters. The Action hook enables you to trigger custom plugin code at specific points during execution. For example, you can trigger a custom function to run after a user registers a user account in WordPress. The Filter hook to modifies text before adding or after retrieving from the database. • Widgets — Create and manage widgets in your plugin. Widgets appear under the Appearance ⇒ Widgets screen and are available to add to any registered sidebar in your theme. The API enables multiple instances of the same widget to be used throughout your sidebars. • Shortcode — Adds shortcode support to your plugin. A shortcode is a simple hook that enables you to call a PHP function by adding something such as [shortcode] to a post or page. • HTTP — Sends HTTP requests from your plugin. This API retrieves content from an external URL or 32 • • • • • • for submitting content to a URL. Currently you have five different ways to send an HTTP request. This API standardizes that process and tests each method prior to executing. Based on your server configuration, the API will use the appropriate method and make the request. Settings — Inserts settings or a settings section for your plugin. The primary advantage to using the Settings API is security. All settings data is scrubbed, so you do not need to worry about cross site request forgery (CSRF) and cross site scripting (XSS) attacks when saving plugin settings. Options — Stores and retrieves options in your plugin. This API features the capability to create new options, update existing options, delete options, and retrieve any option already defined. Dashboard Widgets — Creates admin dashboard widgets. Widgets automatically appear on the Dashboard of WordPress and contain all standard customization features including minimize, drag/ drop, and screen options for hiding. Rewrite — Creates custom rewrite rules in your plugin. This API enables you to add static end-points (/custom-page/), structure tags (%postname%), and add additional feed links (/feed/json/). Transients — Creates temporary options (cached data) in your plugins. This API is similar to the Options API, but all options are saved with an expiration time. Database — Accesses the WordPress database. This includes creating, updating, deleting, and retrieving database records for use in your plugins. 33 WordPress also features pluggable functions. These functions enable you to override specific core functions in a plugin. For example, the wp_mail() function is a pluggable function. You can easily define this function in your plugin and send email using SMTP rather than the default method. All pluggable functions are defined in the /wp-includes/pluggable.php Core WordPress file. You can use some predefined functions during specific plugin tasks, such as when a plugin is activated or deactivated and even when a plugin is uninstalled. Chapter 2, “Plugin Foundation,” covers these functions in detail. When Are Plugins Loaded? Plugins are loaded early in the process when a WordPress powered web page is called. Figure 1-1 shows a diagram of the standard loading process when loading a page in WordPress: FIGURE 1-1 34 Figure 1-1 illustrates the standard process when loading a page in WordPress. The flow changes slightly when loading an admin page. The differences are minor and primarily concern what theme is loaded: admin theme versus your web site theme. AVAILABLE PLUGINS When researching available plugins you need to know where to find WordPress plugins. You can download plugins anywhere on the Internet, but this isn’t always a good idea. As with any software, downloading plugins from an untrusted source could lead to malware injected and compromised plugin files. It’s best to download plugins only from trusted web sites and official sources such as the official Plugin Directory. 35 Official Plugin Directory The first place to start when researching available WordPress plugins is the official Plugin Directory at WordPress.org. The Plugin Directory is located at http://wordpress.org/extend/ plugins/. With more than 10,000 plugins available and well over 100 million plugin downloads, it’s easy to see the vital role plugins play in every WordPress web site. All plugins available in the Plugin Directory are 100% GPL and free to use for personal or commercial use. Popular Plugin Examples Take a look at the five most downloaded WordPress plugins available to get a sense of their diversity: • All in One SEO Pack — Adds advanced search engine optimization functionality to WordPress. Features include custom meta data for all content, canonical URLs, custom post type support, and more! • http://wordpress.org/extend/plugins/ all-in-one-seo-pack/ • Google XML Sitemaps — Generates an XML sitemap of all content for submission to the popular search engines such as Google, Bing, and Ask.com. • http://wordpress.org/extend/plugins/ google-sitemap-generator/ • Akismet — A popular comment spam filter for WordPress. Checks all comments against the Akismet web service to verify whether the comment is spam. • http://wordpress.org/extend/plugins/akismet/ 36 • NextGEN Gallery — Adds advanced image gallery support to WordPress. You can easily create and manage image galleries and slideshows. Galleries can be embedded in posts or pages. • http://wordpress.org/extend/plugins/ nextgen-gallery/ • Contact Form 7 — Adds a contact form to any post or page in WordPress. • http://wordpress.org/extend/plugins/ contact-form-7/ As you can see, the preceding plugins can handle any task. The features added by these plugins are universal and features that most web sites on the Internet should have. Popular Plugin Tags Now you will look at some popular tags for plugins. Plugin tags are just like blog post tags, simple keywords that describe a plugin in the Plugin Directory. This makes it easy to search for existing plugins by tag. Following are popular examples: • Twitter — Everyone loves Twitter for micro-blogging and sharing links. You can find an abundance of Twitter-related plugins for WordPress. • http://wordpress.org/extend/plugins/tags/ twitter • Google — With so many different services and APIs, Google is a popular plugin tag. Everything from Google ads to Google maps have been integrated into a WordPress plugin. 37 • http://wordpress.org/extend/plugins/tags/ google • Widget — Most plugins that include a widget also use the widget tag. This is great for viewing the many different types of widgets available for WordPress. • http://wordpress.org/extend/plugins/tags/ widget Viewing popular plugin tags is a great way to get inspiration when developing new plugins for WordPress. ADVANTAGES OF PLUGINS WordPress offers many advantages to using plugins. You need to understand the advantages to building plugins to truly understand why you should build plugins. This can also help when determining the need for a specific plugin in WordPress. Not Modifying Core One of the main advantages to plugins is the ability to modify the behavior of WordPress without modifying any core files. Core files refer to any file that is a part of the default WordPress installation. Hacking core files can make it difficult to update WordPress when a new version is released. If you made any modifications to a core file, that modification would be overwritten when the update occurs. Keeping WordPress up to date with the latest version is essential in keeping your web site secure. 38 Modifying core files can also lead to an unstable web site. Different areas of WordPress rely on other areas to function as expected. If you modify a core file and it no longer works as expected, it can cause instability and quite possibly break a completely unrelated feature in WordPress. Why Reinvent the Wheel Another advantage to building plugins is the structure that already exists for your plugin. Many of the common features have already been developed and are ready for use in your plugin. For example, you can take advantage of the built-in user roles in WordPress. Using the user roles you can easily restrict your code to execute only if a user is an administrator. Look at an example: <?php if ( current_user_can( 'manage_options' ) ) { //any code entered here will only be executed IF //user is an administrator } ?> As you can see it’s easy to verify a user has proper permissions prior to executing any code in your plugin. You learn about user accounts and roles in Chapter 8, “Users.” 39 As another example, look at sending an email in WordPress. Sure you could create a new function in your plugin to send email, but why? WordPress has a handy function called wp_mail() for sending email. Look at an example: <?php $email_to = '[email protected]What’s New in the Internet Download Accelerator v2.0.1.532 serial key or number?
Screen Shot
System Requirements for Internet Download Accelerator v2.0.1.532 serial key or number
- First, download the Internet Download Accelerator v2.0.1.532 serial key or number
-
You can download its setup from given links:


