
J-Perk 6.05 serial key or number

J-Perk 6.05 serial key or number
As filed with the Securities and Exchange Commission on November 5, 2018.
Registration No. 333-227892
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2 to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
QUALTRICS INTERNATIONAL INC.
(Exact name of Registrant as specified in its charter)
Delaware | 7372 | 47-1754215 |
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
333 West River Park Drive
Provo, Utah 84604
385-203-4999
(Address, including zip code and telephone number, including area code, of Registrant’s principal executive offices)
Ryan Smith
Chief Executive Officer
Qualtrics International Inc.
333 West River Park Drive
Provo, Utah 84604
385-203-4999
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Anthony J. McCusker Bradley C. Weber Goodwin Procter LLP 601 Marshall Street Redwood City, California 94063 650-752-3100 | David Faugno Chief Financial Officer Qualtrics International Inc. 333 West River Park Drive Provo, Utah 84604 385-203-4999 | Dave Peinsipp Charles S. Kim Andrew S. Williamson Cooley LLP 101 California Street, 5th Floor San Francisco, California 94111 415-693-2000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ¨ |
| Accelerated filer | ¨ |
Non-accelerated filer | ý |
| Smaller reporting company | ¨ |
Emerging growth company | ý |
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ý
CALCULATION OF REGISTRATION FEE
Title of Each Class of Securities to be Registered | Amount to be Registered(1) | Proposed Maximum Offering Price Per Share(2) | Proposed Maximum Aggregate Offering Price(2) | Amount of Registration Fee(3) |
Class B Common Stock, par value $0.0001 per share | 23,589,744 | $21.00 | $495,384,624 | $60,041 |
(1) | Includes the 3,076,923 shares of Class B common stock that the underwriters have the option to purchase. |
(2) | Estimated solely for the purpose of computing the amount of registration fee pursuant to Rule 457(a) under the Securities Act of 1933, as amended. Includes offering price of the additional shares that the underwriters have the option to purchase. |
(3) | The registrant previously paid $24,240 of the registration fee with the initial filing of this registration statement. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PROSPECTUS (Subject to Completion)
Issued November 5, 2018
20,512,821 Shares

CLASS B COMMON STOCK
Qualtrics International Inc. is offering 20,512,821 shares of its Class B common stock. This is our initial public offering, and no public market currently exists for our shares. We anticipate that the initial public offering price will be between $18.00 and $21.00 per share.
We have applied to list our Class B common stock on The Nasdaq Global Select Market under the symbol “XM.”
We have three classes of common stock: Class A-1 common stock, Class A-2 common stock, and Class B common stock. The rights of the holders of Class A-1 common stock, Class A-2 common stock, and Class B common stock are different with respect to voting, conversion, and transfer rights. Each share of Class B common stock is entitled to one vote. Each share of Class A-1 common stock is entitled to ten votes. Each share of Class A-2 common stock is also entitled to ten votes, but in the event that the total voting power of the Class A-2 common stock represents less than 51% of the total voting power of all our outstanding capital stock, then the voting power of each share of Class A-2 common stock will be automatically increased so that the Class A-2 common stock collectively holds 51% of our total voting power. In such event, the voting power of shares of Class B common stock (the shares being issued in this offering) and Class A-1 common stock will be automatically and proportionately reduced. Upon completion of this offering, approximately 87% of our Class A-2 common stock will be held by Grandview Holdings, LLC, the managers of which are our founders, including our Chief Executive Officer (who is also a director), our President (who is also a director), and our former President (who is also a director). This means that, for the foreseeable future, investors in this offering and holders of our Class B common stock in the future will not have a meaningful voice in our corporate affairs and that the control of our company will be concentrated with Grandview Holdings, LLC and the other holders of our Class A-2 common stock. See the section titled “Risk Factors—Risks Related to Ownership of Our Class B Common Stock and this Offering” for additional information.
We are an “emerging growth company” as defined under the federal securities laws and, as such, may elect to comply with certain reduced public company reporting requirements for this and future filings. Investing in our Class B common stock involves risk. See “Risk Factors” beginning on page 18.
PRICE $ A SHARE |
| Price to |
| Underwriting Discounts and |
| Proceeds to |
Per Share | $ |
| $ |
| $ |
Total | $ |
| $ |
| $ |
(1) | See “Underwriters” for a description of the compensation payable to the underwriters. |
We have granted the underwriters the right to purchase up to an additional 3,076,923 shares of Class B common stock solely to cover over-allotments, if any.
The Securities and Exchange Commission and state regulators have not approved or disapproved of these securities, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of Class B common stock to purchasers on , 2018.
MORGAN STANLEY | GOLDMAN SACHS & CO. LLC | |||||||
BARCLAYS | RBC CAPITAL MARKETS |
| JEFFERIES | DEUTSCHE BANK SECURITIES | BMO CAPITAL MARKETS | |||
KEYBANC CAPITAL MARKETS |
| RAYMOND JAMES | CANACCORD GENUITY | BAIRD | BTIG | |||
, 2018



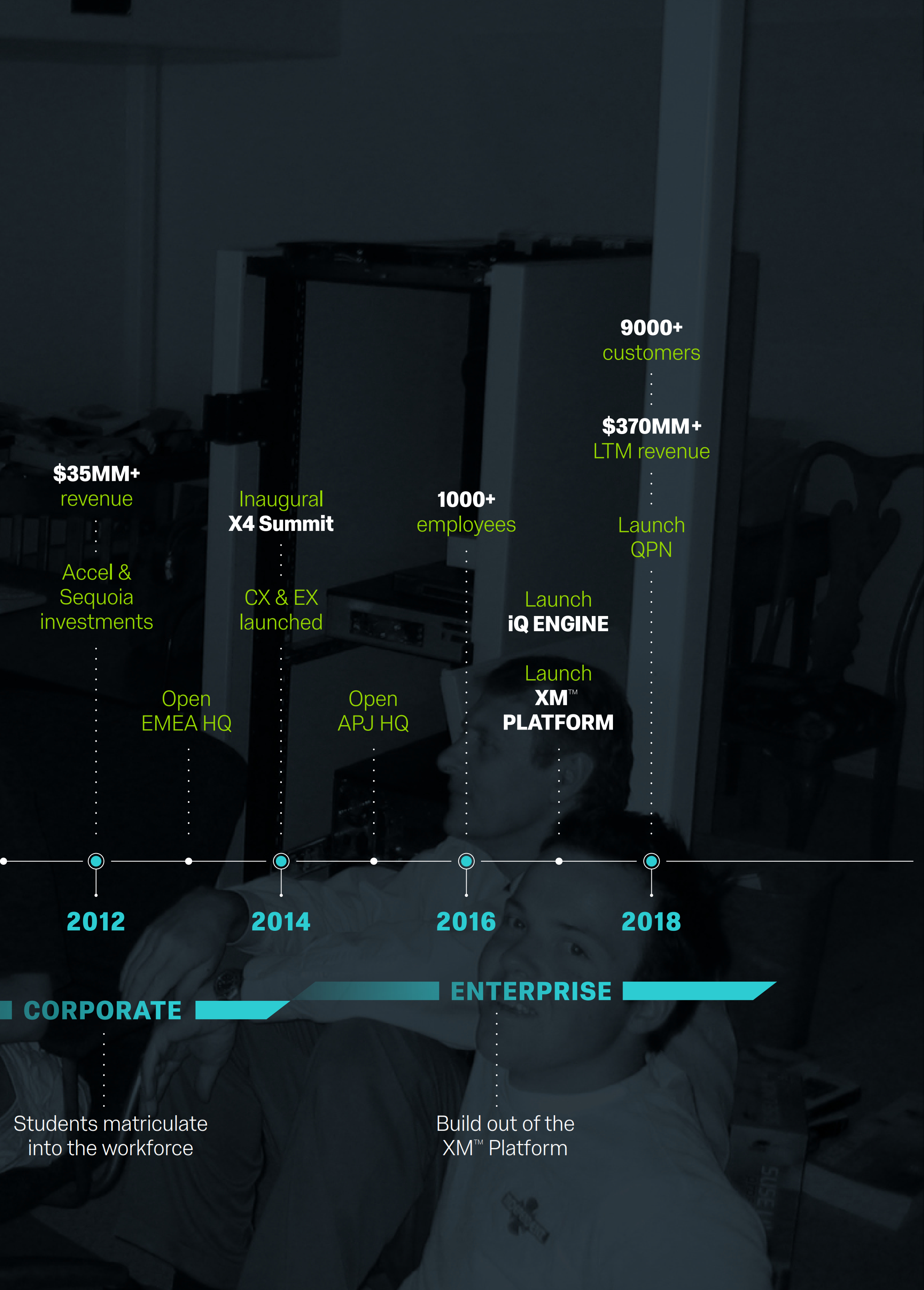
TABLE OF CONTENTS
Through and including , 2018 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
We have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses we have prepared. We and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We are offering to sell, and seeking offers to buy, shares of Class B common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the Class B common stock.
For investors outside the United States: Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of Class B common stock and the distribution of this prospectus outside of the United States.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our Class B common stock, you should carefully read this entire prospectus, including our consolidated financial statements and the related notes included elsewhere in this prospectus and the information set forth under the sections titled “Risk Factors,” “Special Note Regarding Forward-Looking Statements,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Unless the context otherwise requires, we use the terms “Qualtrics,” “company,” “our,” “us,” and “we” in this prospectus to refer to Qualtrics International Inc. and its consolidated subsidiaries. Our fiscal year ends December 31.
QUALTRICS INTERNATIONAL INC.
Overview
Qualtrics has pioneered a new category of software that enables organizations to succeed in today’s experience economy. Our mission is to help organizations deliver the experiences that turn their customers into fanatics, employees into ambassadors, brands into religions, and products into obsessions.
We Live in an Experience Economy
Today, organizations thrive or fail based on the experiences they deliver. In a world of abundant choice, experiences differentiate brands and products, and foster customer and employee loyalty. Great experiences drive customer loyalty, upsell and expansion, employee engagement, brand quality, improved retention and referral, and ultimately, greater shareholder value. Conversely, unfavorable experiences lead to increased churn, lower productivity, diminished competitiveness, and value destruction. With the advent of digital communication channels, favorable or unfavorable experiences can be shared instantly and spread virally, amplifying these impacts and raising the stakes for organizations of all types and sizes.
Executives Must Own All Dimensions of Experience
In this environment, C-level executives are increasingly accountable for issues that transcend basic product and service quality and encompass all of the dimensions that surround those offerings. This extends to thousands of often subtle factors that determine the quality of experiences their organizations deliver, including company culture, speed, convenience, attentiveness, design, and ease of use. We believe that customer, employee, brand, and product experience represent the four vital signs of organizational well-being and that executives are now measured on their performance across these domains. Customer and employee expectations are high, setting up the potential for significant gaps between actual and anticipated experiences. Yet, executives often lack the tools to understand, assess, and take decisive action to address these “experience gaps” as they arise. Today, disruptive start-ups and other businesses flourish by identifying such gaps and designing experiences that attack these blind spots of incumbents.
Organizations Need to Address Experience Directly, in the Moment
While organizations have traditionally deployed consultants or other third parties to gather data about customer and employee satisfaction, the increasing centrality, complexity, and nuance of delivering great experiences has compelled C-level executives to seek the capability to understand and take ownership of these matters directly and in real time. Given the immediate nature of experience, there is also a strong desire to allow individuals at every level in an organization to comprehend changes in experience quality and empower them to act decisively when it matters most.
We Pioneered Experience Management to Power the Experience Economy
We have created a new category of software, Experience Management, or XM™, which enables organizations to address the challenges and opportunities presented by the experience economy. XM™ allows organizations to accomplish the following:
• | Comprehensively gather and analyze a new class of data, Experience Data, or X-data™, that is richer, more immediate, and more salient to understanding quality of experience than traditional operational data, or O- |
data™, which comes from sources such as customer relationship management, or CRM, enterprise resource planning, or ERP, human capital management, or HCM, Customer Service, and Marketing Automation systems. X-data™ is the human factor data — individual beliefs, emotions, and intentions — collected across multiple channels through which customers and employees engage with an organization.
• | Go beyond an assessment of what is happening within organizations to an understanding of why trends are emerging in the moment. |
• | Address experience holistically, unifying information and insights from customers, employees, and partners and recognizing the operational linkages between the sentiment of these constituencies. |
• | Become more predictive and proactive, closing feedback loops, and turning insight into real-time action to prevent and close experience gaps where they exist. |
• | Democratize and own analysis and decision making across the organization by delivering powerful capabilities in a simple, easy-to-use product. |
In today’s experience economy, we believe that XM™ is more critical to improving customer experience than CRM, more influential upon employee experience than HCM systems, and more important to enhancing brand experience than Marketing Automation. Consequently, we believe that XM™ represents a vast, rapidly growing, and underpenetrated market opportunity, and we estimate our total addressable market to be approximately $44 billion in 2018.
Our History Uniquely Positioned Us to Create and Lead XM™
Our history uniquely positioned us to pioneer and develop this new XM™ category. Founded in 2002 with the goal of solving the most complex problems encountered by the most advanced academic researchers, we were forged in an environment that required rigorous analytical methods, ease of use, the versatility to address the broadest range of inquiries, and the scalability to reach millions of touch points globally. Our leading presence with academic institutions has introduced millions of students to Qualtrics and allowed them to become proficient in the use of our software. As these students have migrated into the workplace, they have often brought us with them, spawning a whole new class of commercial customers and developing new use cases for our XM™ Platform. Led by these customers, we evolved beyond our traditional research product offering to develop our platform, which incorporates our core research capability and is also designed to specifically address customer, employee, brand, and product use cases. Taken together, we believe that this platform provides a System of Action for organizations to monitor and act upon the vital signs that drive performance in any organization.
Our Platform Defines All the Elements of Experience Management
Our XM™ Platform is purpose-built to help organizations collect feedback and data across the four vital signs of a business: Customers, Employees, Brand, and Product. XM™ transforms that data into insight, and drives action to create value. The key elements of our platform include:
• | Research Core — A collection of powerful, flexible research tools to build and distribute data collection systems, aggregate and analyze data, build reports, and draw insight from data. Research Core is designed specifically to instrument, gather, and index human factor data in any format through any channel. Users can synthesize and identify trends within minutes and immediately dig deeper into any data point to extract additional insight. |
• | Customer Experience (CX) — Enables a deep understanding of customer sentiment throughout every customer journey allowing organizations to monitor, measure, and take action where there may be any experience gaps, with a focus on identifying specific challenges and designing solutions to remediate issues and improve satisfaction in the moment. |
Peptidomimetics Protocols
Introduction
In Peptidomimetics Protocols, Wieslaw Kazmierski assembles a state-of-the-art collection of detailed synthetic procedures that lead to a variety of scaffolds, turn mimetics, peptide-bond replacements, and enzyme inhibitors. Topics range from syntheses of unusual amino acids, to the use of a variety of linear and heterocyclic scaffolds in place of the peptide backbone. Important chemical procedures and methods include the transient protection of charged peptides as neutral prodrugs for improved blood-brain penetration and the replacement of otherwise labile peptide bonds with heterocyclic rings, olefins and fluoroolefins, and ketomethylenes. Synthetic protocols towards the transition-state mimics and reactive "warheads," applicable in enzyme inhibitors, are also disclosed.
Peptidomimetics Protocols is the first book devoted to the practical synthetic preparation of peptide mimetics. Written by both academic and industrial synthetic organic and medicinal chemists, this book provides highly practical synthetic procedures for the generation of key peptide mimetics, and so immediately becomes a must-have desk reference and guide for all medicinal and pharmaceutical chemists engaged in the discovery and development of pharmaceuticals today.
Editors and affiliations
- 1.Glaxo Wellcome., Inc.Research Triangle Park
Bibliographic information
- DOIhttps://doi.org/10.1385/0896035174
- Copyright InformationHumana Press 1999
- Publisher NameHumana Press
- eBook PackagesSpringer Protocols
- Print ISBN978-0-89603-517-1
- Online ISBN978-1-59259-605-8
- Series Print ISSN1543-1894
- Series Online ISSN1940-6037
- Buy this book on publisher's site
Galectin-3 modulates epithelial cell adaptation to stress at the ER-mitochondria interface
Abstract
Cellular stress response contributes to epithelial defense in adaptation to environment changes. Galectins play a pivotal role in the regulation of this response in malignant cells. However, precise underlying mechanisms are largely unknown. Here we demonstrate that Galectin-3, a pro and anti-apoptotic lectin, is required for setting up a correct cellular response to stress by orchestrating several effects. First, Galectin-3 constitutes a key post-transcriptional regulator of stress-related mRNA regulons coordinating the cell metabolism, the mTORC1 complex or the unfolded protein response (UPR). Moreover, we demonstrated the presence of Galectin-3 with mitochondria-associated membranes (MAM), and its interaction with proteins located at the ER or mitochondrial membranes. There Galectin-3 prevents the activation and recruitment at the mitochondria of the regulator of mitochondria fission DRP-1. Accordingly, loss of Galectin-3 impairs mitochondrial morphology, with more fragmented and round mitochondria, and dynamics both in normal and cancer epithelial cells in basal conditions. Importantly, Galectin-3 deficient cells also display changes of the activity of the mitochondrial respiratory chain complexes, of the mTORC1/S6RP/4EBP1 translation pathway and reactive oxygen species levels. Regarding the ER, Galectin-3 did not modify the activities of the 3 branches of the UPR in basal conditions. However, Galectin-3 favours an adaptative UPR following ER stress induction by Thapsigargin treatment. Altogether, at the ER-mitochondria interface, Galectin-3 coordinates the functioning of the ER and mitochondria, preserves the integrity of mitochondrial network and modulates the ER stress response.
Introduction
Eukaryotic cells are partitioned into discrete organelles endowed with specific functions. For example, protein synthesis, folding, secretion and degradation take place at the endoplasmic reticulum (ER), whereas cellular bioenergetics occurs at the mitochondria. Cell homeostasis depends on the ability of these distinct organelles to communicate and to exchange cargos. Among them, the ER appears especially important since it can develop close contacts with most organelles, notably the mitochondria1. Interestingly, the ER-mitochondria interactions which are commonly referred to as mitochondria-associated membranes (MAM) regulate important cellular processes such as mitochondrial biogenesis and metabolism, calcium signalling or lipid biosynthesis2,3. When the protein synthetic load overcomes the ER folding capacities, a state called ER stress occurs. To restore ER homeostasis, a network of transduction pathways named unfolded protein response (UPR) is activated4. Collectively, these pathways act to reduce protein synthesis and to increase ER folding and ER-associated degradation (ERAD) capacities. However, when this adaptative response fails the UPR evolves towards apoptosis.
Galectin-3 is a β-galactoside-binding lectin, encoded by LGALS3 gene in humans, which contains a C-terminal carbohydrate recognition domain (CRD) responsible for interactions with glycolipids or glycoproteins and a low complexity domain which allows interactions with the CRD and other partners5,6. Moreover, despite the absence of a canonical RNA-binding domain, Galectin-3 is a non-classic RNA-binding protein (RBP) able to stabilise mucin MUC4 mRNAs in cancer cells7. Galectin-3 is highly expressed by epithelial cells and plays important roles in the organisation of renal and intestinal cells. Although Galectin-3-KO mice are viable in controlled conditions, loss of Galectin-3 leads to morphological abnormalities of the epithelial cells as well as perturbation of the biosynthetic pathway8,9,10. Galectin-3 is a soluble protein which is synthesised on free ribosomes and thus bypasses the classical ER-Golgi pathway for its secretion in the extracellular medium. Indeed, premature binding of Galectin-3 with its ligands which are major components of the ER lumen would cause aggregation and perturb the secretory pathway11,12. While being synthesised in the cytosol, Galectin-3 associates with various organelles, such as carrier vesicles or endosomes13. At the mitochondria level, Galectin-3 prevents the cytochrome-c release and ensures mitochondrial integrity14,15. However, it is currently unknown whether these mitochondrial effects depend on Galectin-3 ability to modulate mitochondria-ER interactions in epithelial cells.
In the present study we first aimed to obtain a global view of the post-transcriptional regulatory action of Galectin-3 in epithelial cells. To this end, we combined whole transcriptome stability analysis with mRNA and protein quantification. We showed that Galectin-3 regulates the stability of subsets of mRNAs which share similar functions notably cell metabolism, cell death and stress response pathways. By coupling imaging and biochemical approaches, we showed that Galectin-3 localises at the ER-mitochondria interface where it preserves the integrity of the mitochondrial network and modulates the cellular bioenergetics and the UPR.
Results
Gal-3 regulates the half-life of subsets of mRNAs with shared functions
We first aimed to obtain a global view of the action of Galectin-3 as a post-transcriptional regulator in epithelial cells. For that purpose, we used two models deriving from the human pancreatic cancer cell line T3M-4, control Sc cells expressing high levels of Galectin-3 and a representative mutant clone (Sh1 called Sh cells thereafter) where Galectin-3 expression was stably knocked-down by shRNA (Fig. S1). Using actinomycin D and RNA-Seq, we performed a whole-transcriptome stability analysis. We built the decay curve for 9444 transcripts in both models using the most appropriate kinetic model. In all, 3356 transcripts with a decay curve characterised by a r2 ≥ 0.9 were selected for further analysis. Rapidly decaying mRNAs (t1/2 < 3.0 h) were excluded as the experimental time-points did not allow reliable calculation of their half-lives. Therefore, the total number of RNAs analysable was 2275 (Fig. 1a). A total of 440 mRNAs showed a ≥2-fold difference in their half-life between Sc and Sh cells (Table S1). The vast majority (385 mRNAs) were stabilised by Galectin-3 whereas only 55 mRNAs were destabilised by Galectin-3 (Fig. 1a, b and Table S1). To further identify the biological processes and the organelles involved, the set of mRNAs whose stability was modified by Galectin-3 (n = 440) was submitted to an analysis by the Gene Set Enrichment Analysis (GSEA) tool (Fig. 1c, d). Focusing on cellular component, the mitochondria and the ER were significantly enriched in proteins whose mRNAs are post-transcriptionally regulated by Galectin-3 (Fig. 1c, Table S2). Using Hallmark as a reference collection, 13 biological pathways were significantly enriched in our data set, several previously unrelated to Galectin-3 such as metabolic pathways (i.e. glycolysis, fatty acid metabolism) and the UPR (Fig. 1d and Table S2). Regarding UPR, twelve mRNAs stabilised by Gal-3 were classified in this category by GSEA (in black). Twelve additional mRNAs (in blue) were assigned to UPR based on the Gene Ontology (GO) annotation of the gene product as determined with AmiGO (Fig. 1d). To check whether changes of the mRNA half-life influence mRNA levels, we evaluated by qPCR the steady state mRNA levels of 7 mRNA from the UPR category (Fig. 1d, underlined) and stabilised by Galectin-3. All but one mRNA showed a significant reduction of expression in Sh cells by comparison with Sc cells (Fig. 1e). Moreover, we studied protein expression of four mRNA stabilised by Galectin-3, namely PARN, XPOT, USP14 and SEL1L in basal state. Our results showed that the protein levels of USP14 and SEL1L, both involved in ERAD, were significantly higher in Sh cells whereas a similar trend existed for PARN and XPOT (Fig. 1f). Thus Galectin-3, as a non-conventional RBP, modulates selectively the half-life, levels and translation of mRNAs whose encoded proteins share similar biological functions (RNA regulons).
Localisation of Galectin-3 at the ER-mitochondria interface
Since Galectin-3 post-transcriptionally regulates several genes involved in mitochondria and/or ER functioning, we next wondered whether Galectin-3 could localise at the ER-mitochondria interface. Using high-resolution fluorescence microscopy, KDEL as a proxy of the ER network and Mitotracker to stain the mitochondria (Fig. 2a), we showed that, although the ER and mitochondria display by themselves 12% of contacts, ~13% of the ER-mitochondria interfaces associate with Galectin-3 in pancreatic Sc cells (Fig. 2b). Conversely, 4% of total Galectin-3 associates with this area (as expected for a highly expressed lectin which associates with diverse subcellular compartments13,16). In addition, live microscopy using Mitotracker and Gal-3 dsRed construct validated the dynamic association between Galectin-3 and the mitochondria over time (Movie S1). Immunogold staining of human intestinal Caco2 cells coupled with transmission electron microscopy (TEM) revealed that Galectin-3 does associate with the ER complex. However, this association is transient, and specifically occurs at contact sites between the ER and the mitochondria (Fig. 2c, yellow circles and arrows). Finally, we performed density gradient centrifugations to collect subcellular fractions enriched in mitochondria, MAMs and ER, according to a reference method. We confirmed that a pool of intra-cellular Galectin-3 is present in MAM-enriched fraction in both Caco2 and pancreatic Sc cells (Fig. 2d). The presence of Galectin-3 in the ER fraction could be explained by its possible contamination by lysosomes. Interestingly, among the interacting partners of Galectin-3 identified in Caco-2 cells by immunoprecipitation (IP) coupled with mass spectrometry analysis, seven (≈4% of the whole) are exclusively located at the ER membrane such as TBL2, a regulator of ER stress response, and 13 in the mitochondria such ATP5J (Table S3 and Fig. S2). To conclude, by combining several approaches we show that Galectin-3 is found at MAMs thus suggesting a dedicated function of Galectin-3 at that level.
Impact of Galectin-3 on mitochondria morphology, dynamics and bioenergetics
The ER-mitochondria interface constitutes a key hub for the ER stress response and mitochondria bioenergetics17,18. We then hypothesised that Galectin-3 may be instrumental there for cell adaptation to stress and/or mitochondrial homeostasis. First, we studied the impact of Galectin-3 on the morphology and behaviour of mitochondria. Ultrastructural analyses performed in WT and Lgals3−/− mouse enterocytes (Fig. 2e) showed that loss of Galectin-3 provokes the formation of enlarged and swollen mitochondria whereas in wild-type cells mitochondria display the classical “stick shape”. As expected, image analysis confirmed an increased maximum diameter in Galectin-3 deficient versus control mouse enterocytes (Fig. 2f). Similarly, ultrastructural analysis of the mitochondrial network in Sh cells revealed irregular mitochondrial shape in comparison with controls Sc cells (Fig. 2g). In addition, numerous degradative compartments appear in close proximity of mitochondria in Sh cells. We concluded that Galectin-3 is required for maintenance of typical mitochondrial morphology in epithelial cells. Second, we analysed the architecture of the mitochondrial network (Fig. 3a). In dense cultures, analysis of the images with the MiNA toolset19 enabled to calculate the values of mitochondrial network 2D parameters. The mitochondrial footprint (Fig. 3a, b), the number of branches per network and the summed branch length (Fig S3a) were significantly decreased in Sh cells versus control Sc cells. These results show that, in the absence of Galectin-3, the mitochondrial network exhibits a less tubular shape but instead mitochondrial fragmentation (Fig. 3a, d, e). Alterations of the basal ER stress level in Sh cells could be responsible for this phenotype. Along this line, we induced acute ER stress response by treating Sc and Sh cells with 250 nM Thapsigargin alone, or in combination with either GSK2606414, a potent and selective inhibitor of PERK, or ISRIB, a PERK-independent stabiliser of eIF2α dephosphorylation. Thapsigargin in Sc cells induced a significant decrease of mitochondrial footprint (Fig. 3b), of the number of branches per network and of the summed branch length (Fig. S3), leading to mitochondrial characteristics very similar to those of Sh cells. As expected, combined treatment with GSK2606414 or ISRIB partially reversed mitochondrial morphology in Sc cells. On the contrary, in the absence of Galectin-3, treatment with Thapsigargin very negligibly altered the mitochondrial footprint and branching. Moreover, combination with GSK2606414 or ISRIB had no significant effect compared to Thapsigargin treatment alone, and did not revert mitochondrial morphology, indicating that mitochondrial fragmentation in Sh cells is independent of UPR. Hence, the loss of Galectin-3 silencing may provoke a perturbation of the mitochondrial dynamics, most probably in relation with fission/fusion events. Therefore, we analysed the behaviour of DRP-1 which is the central modulator of mitochondrial fission20. Phosphorylation of Drp-1 at Ser637 prevents its translocation from the cytosol to the mitochondria and its fission activity. By contrast, pSer616 Drp-1 stimulates mitochondrial fission21. Here we observed by immunofluorescence that pSer637 Drp-1 expression was higher in Sc than Sh cells, a trend further confirmed by western blotting analysis of mitochondrial extracts (Fig. 3d, e). By contrast, pSer616 Drp-1 was highly expressed in Sh cells and frequently co-localised with Tom20, a mitochondrial marker. Total Drp-1 level was also significantly increased in Sh cells (Fig. S3b). Altogether, these data testify a mitochondrial pro-fission phenotype in the absence of Galectin-3. Moreover, to test mitochondrial plasticity, we performed live cell imaging and mitochondrial motility was assessed by particle-tracking analysis of Mitotracker-labelled mitochondria in Sc (Movie S2) and Sh cells (Movie S3). Whatever the parameter used (i.e. speed or distance), the motility of mitochondria was significantly decreased in absence of Galectin-3 (Fig. 4a, b). Altogether, these results reveal that Galectin-3 influences the mitochondrial dynamics/homeostasis by inhibiting the fission process and promoting the motility instead.
To determine whether morphological abnormalities may impinge on mitochondrial activity, the cell respiration was studied by oxygraphy using O2k (OROBOROS instruments). Results showed that the activities of mitochondrial Complex I (NADH dehydrogenase) and Complex IV (cytochrome C oxydase) were significantly higher in Sh versus control pancreatic Sc cells (Fig. 5a). The activities of Complex II and III did not differ between Sh and control Sc cells. The electron transfer system (ETS) measured after addition of FCCP, an uncoupling agent, was significantly higher in Sh than control Sc cells (Fig. 5a). By contrast no significant difference was observed for Complex V nor ETS measured in coupled conditions (C ETS). In intestinal cells, no significant differences in the activities of Complexes I–V and ETS were observed between H3 and NT control cells, probably because of the high SD values. However, despite the versatility of Caco2 cells, Galectin-3 depleted cells presented a reproducible and significant increase in C ETS when compared to control cells (p = 0.0169).
Since oxidative phosphorylation (OXPHOS) is the main contributor to ATP cellular production and occurs downstream of the glycolysis, we hypothesised that this inhibitory effect of Galectin-3 may induce a cellular metabolic stress. We therefore measured extracellular lactate levels as a proxy of the glycolytic activity. Lactate levels were higher in Sc versus Sh pancreatic cells (Fig. 5b, p < 0.05), whereas no difference occurred between Galectin-3-silenced Caco2 cells and parental cells. However, the ADP/ATP ratio, which is inversely related to the cell energy status, was not significantly lower in Sh than control Sc cells. Thus, in the presence of Galectin-3 the mitochondrial OXPHOS is less efficient but no metabolic stress is observed. Leakage of electrons from the mitochondrial electron transport chain results in the generation of reactive oxygen species (ROS) mainly at the level of complexes I and III22. By flow cytometric analysis of MitoSOX® Red-stained populations, we herein showed that the number of cells with positive mitochondria was not different between Sc and Sh cells (Figs. 5c and S4). However, there was a higher percentage of high ROS cells in Sh than in control Sc cells (44% vs 16.5%, p < 0.0001) in agreement with a higher OXPHOS activity in the mitochondria of Galectin-3 silenced cells.
To explain these differences in mitochondria dynamics and bioenergetics, we wondered whether Galectin-3 might affect the expression of components of the respiratory chain post-transcriptionally. In this respect, we identified two species of interest among the mRNAs destabilised by Galectin-3 (Table S1). First, PDSS2 which encodes DLP1, a protein involved in the biosynthesis of coenzyme Q which promotes electron transfer between complexes II and III23, and GUF1 alias EF4 which encodes a protein of the mitochondrial inner membrane important for complex IV organisation and activity24. By western blotting (Fig. S5), we showed that Galectin-3 did not influence the expression of DLP1 or GUF1. The same was observed for ISCU, a scaffold protein involved in the assembly of Fe–S cluster found in complexes I, II and III. However, among the interacting partners of Galectin-3 identified by co-immunoprecipitation we did find ICSU, and also other members of mitochondrial complexes such as UQCRC2 (Complex III) or the F0 complex of ATP Synthase (Table S3).
Gal-3 selectively controls mRNA translation through mTORC1
Since mTORC1 is a global sensor of the cell nutrient status we wondered whether Galectin-3 expression may influence mTORC1 activity due to its negative impact on OXPHOS. Addition of fetal calf serum (FCS), an activator of mTORC1, in serum-starved cells failed to induce S6RP and 4EBP1 phosphorylation in control cells by contrast with Sh cells (Fig. 6a). In agreement, a trend towards higher levels of phosphorylated mTOR at Ser2448 was observed in Sh cells vs control cells but the phospho-mTOR/mTOR ratio was not different between Sh and Sc, probably because a decrease of total mTOR occurred in Sc cells (Figs. 6a and S6). No difference was observed for the AMPK pathway between Sc and Sh cells, in accordance with the absence of metabolic stress reported earlier (Fig. 5b). Thus the presence of Galectin-3 decreases S6RP and 4EBP1 activity in part by reducing mTORC1 activation. Since phosphorylation of both S6RP and 4EBP favours mRNA translation initiation, Galectin-3 inhibitory effect may induce a blockage of mRNA translation in pancreatic cancer cells in usual culture conditions (with FCS). To validate this point, we prepared protein extracts and total RNA simultaneously and measured the protein levels by western blot and mRNA levels by qPCR of four genes representative of the mTORC1 and fatty acid metabolism GO categories whose mRNAs are stabilised by Galectin-3. Whereas GBE1 and SLC2A3 mRNA levels were higher in Sc cells than in Sh cells, the corresponding protein levels were unchanged or decreased in Sc versus Sh cells, respectively (Fig. 6b). Similarly, ACOT2 protein levels were decreased in Sc mitochondrial extracts whereas its mRNA levels were similar between Sc and Sh cells. Thus, the differential expression of transcript and protein observed for GBE1, ACOT2 and SLC2A3 but also for USP14 and XPOT (Fig. 1f) suggests that, in presence of Galectin-3, the translation of a fraction of these 5 mRNAs is blocked (Fig. 6c). A second category of mRNAs is represented by ETFDH whose mRNA and protein levels were unaffected by Galectin-3 expression (Fig. 6). In conclusion, Galectin-3 appears to reprogram selectively the translatome of pancreatic cancer cells in basal conditions through the control of S6RP and 4EBP1 activity.
Gal-3 promotes an early transactivation of ATF6- and Xbp-1s target genes following ER stress induction
In mammalian cells, three canonical ER resident transmembrane proteins (IRE1, PERK and ATF6) act as ER stress sensors and activate three pathways (Fig. 7a). mRNA expression levels of 84 key UPR genes were then monitored by PCR arrays, at baseline (T0) and 1, 4 and 16 h following ER stress induction by treatment with 250 nM Thapsigargin. Levels of 31 mRNAs showed significant variations, among which 26 were significantly increased at early phases of cell response to Thapsigargin treatment whereas 5 were diminished in control Sc cells versus Sh cells respectively (Fig. 7b). Most of these mRNA inductions in Sc cells occurred at steady state (38.5%) or after 1 h of Thapsigargin treatment (61.5%). Based on promoter structure analysis25, the 31 mRNAs which showed differential expression between Sc and Sh cells were classified according to the specific contribution of ATF4, ATF6(N) and Xbp-1s transcription factors for their expression (Fig. 7b). Interestingly, seven mRNAs correspond to ATF6(N)-target genes, three mRNAs to Xbp-1s-target genes, three mRNAs to ATF6(N) and Xbp1-s target genes, whereas only one mRNA corresponds to ATF4-target genes. Next we decided to study the impact of Galectin-3 on the three UPR branches at the protein level. As soon as 1 h of Thapsigargin treatment, the ratio cleaved ATF6/full length AT6 protein was significantly increased in control Sc cells, demonstrating an engagement of the ATF6 arm (Fig. 7c). Hence, we asked whether this could translate into early transactivation of specific ATF6 targets. Indeed, we observed that 1 h after Thapsigargin treatment ATF6B and MBTPS1 mRNA levels (Figs. 7c and S7) were significantly increased in Sc versus Sh cells. Regarding the IRE1 branch (Fig. 7d), Xbp-1s protein expression was increased in control Sc cells as early as 1 h after Thapsigargin addition (p < 0.05 versus baseline) whereas in Sh cells activation was delayed. Maximal induction of Xbp-1s occurred at 4 h time course in both cell lines (Figs. 7d and S7). Accordingly, some XBP-1s-dependent targets such as ERP44, EDEM1 (Fig. 7d) or HERPUD1 (Fig. S7) were also significantly up-regulated in control Sc cells 1 h after Thapsigargin treatment. Finally, concerning the PERK arm (Fig. 7e), phosphorylation of eif2α was delayed in control Sc cells as was ATF4 protein expression. In fact, ATF4 protein levels were significantly higher as early as 1 h after Thapsigargin addition only in Sh cells (p < 0.05 versus baseline, Fig. S5). Since ATF4 mRNA levels did not vary significantly during Thapsigargin treatment, ATF4 protein expression results from a phospho-eif2α-dependent translation reprogramming. Taken together, these data show that Galectin-3 favours an early activation of the ATF6 and IRE/Xbp1s arms of the UPR resulting in an increase of the ER protein folding (ERP44, CALR) as well as ERAD pathway (HERPUD1) capacities. Moreover, Galectin-3 also delays ATF4 expression during an experimental ER stress. Finally, we studied the influence of Galectin-3 on cell viability during a prolonged Thapsigargin treatment (>16 h). By live and dead assay, we demonstrated that the mortality of control Sc cells was significantly higher than those of Sh cells at 24 and 48 h of the treatment (Fig. 7f).
What’s New in the J-Perk 6.05 serial key or number?
Screen Shot
System Requirements for J-Perk 6.05 serial key or number
- First, download the J-Perk 6.05 serial key or number
-
You can download its setup from given links:


